* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Antibody responses of variable lymphocyte receptors in the lamprey
Duffy antigen system wikipedia , lookup
Immunocontraception wikipedia , lookup
Immune system wikipedia , lookup
Anti-nuclear antibody wikipedia , lookup
Psychoneuroimmunology wikipedia , lookup
DNA vaccination wikipedia , lookup
Lymphopoiesis wikipedia , lookup
Molecular mimicry wikipedia , lookup
Innate immune system wikipedia , lookup
Adaptive immune system wikipedia , lookup
Adoptive cell transfer wikipedia , lookup
Cancer immunotherapy wikipedia , lookup
Polyclonal B cell response wikipedia , lookup
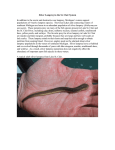
© 2008 Nature Publishing Group http://www.nature.com/natureimmunology ARTICLES Antibody responses of variable lymphocyte receptors in the lamprey Matthew N Alder1,2, Brantley R Herrin1,2, Andrea Sadlonova1,2, Cecil R Stockard3, William E Grizzle3, Lanier A Gartland1,2, G Larry Gartland2, Jeremy A Boydston2, Charles L Turnbough2, Jr & Max D Cooper1–4 Lamprey and hagfish, the living representatives of jawless vertebrates, use genomic leucine-rich-repeat cassettes for the combinatorial assembly of diverse antigen receptor genes encoding variable lymphocyte receptors of two types: VLRA and VLRB. We describe here the VLRB-bearing lineage of lymphocytes in sea lamprey. These cells responded to repetitive carbohydrate or protein determinants on bacteria or mammalian cells with lymphoblastoid transformation, proliferation and differentiation into plasmacytes that secreted multimeric antigen-specific VLRB antibodies. Lacking a thymus and the ability to respond to soluble protein antigens, lampreys seem to have evolved a B cell–like system for adaptive humoral responses. Jawless vertebrates have an adaptive immune system that rivals that of humans in the extent of its clonal diversity, wherein each lymphocyte expresses a unique anticipatory receptor for antigen1–3. However, the diverse antigen receptors in lamprey and hagfish, the only living representatives of the jawless vertebrates (agnathans), are constructed with building blocks that differ from the immunoglobulin variable, diversity and joining segments used for the construction of human T cell antigen receptors and B cell antigen receptors. The variable lymphocyte receptors (VLRs) expressed by lamprey and hagfish lymphocytes are composed of leucine-rich-repeat (LRR) protein segments and an invariant stalk region that is tethered to the lymphocyte surface by glycosylphosphatidylinositol linkage. The LRR segments in VLRs vary in number and amino acid sequence. The sequence variations are concentrated in the b-sheets that form the inner concave surface of the crescent-shaped LRR proteins, which suggests that this is the antigen-binding surface2,4. Two germline VLR genes, VLRA and VLRB, have been identified in lamprey and hagfish1,3,5. Each is incomplete by virtue of having coding sequences for only the invariant 5¢ and 3¢ ends of the VLR molecules. Multiple LRR cassettes flank these germline VLR genes, and these encode all or a portion of the LRR modular units needed for VLR gene completion. During lymphocyte differentiation, pieces of the flanking LRR sequences are ‘stitched’ into the germline VLR gene in a sequential way1,2,5,6. This is achieved by a gene-conversion mechanism in which short stretches of nucleotide sequence homology between donor and recipient VLR gene elements serve as anchorage sites for stepwise extension, culminating in the completion of a mature VLR gene. The gene conversion process begins on either the 5¢ or 3¢ end of the germline VLR gene, is confined to one allele and may involve an AID-APOBEC cytidine deaminase family member. The dozens of flanking LRR cassette sequences seem to be randomly selected for use in VLR gene assembly, except that short stretches of homology are required for anchorage of the donor cassette to the acceptor region of the VLR gene being constructed. These gene assembly features ensure the generation of an extensive repertoire of lymphocytes, whose potential diversity is estimated to be over 1 1014 for the agnathan VLRs2,5. Long before discovery of the VLRs, lamprey and hagfish were found to produce agglutinins to particulate antigens7–14. It has been shown that lamprey produce soluble antigen-specific VLRs in response to immunization with Bacillus anthracis exosporium2, the outmost layer of spores of the bacterium that causes anthrax15. Here we have revisited the lamprey humoral response to define requirements for the antigen induction of VLRB antibody responses as well as the molecular composition of VLRB antibodies and their potential protein- and carbohydrate-binding specificities. We have also explored the cellular basis for the humoral response by using mouse monoclonal antibodies to the invariant stalk region of VLRB molecules to characterize the morphology, distribution, proliferation and differentiation of VLRB-producing lymphocytes in the lamprey. RESULTS Production of VLRB antibodies to heterologous erythrocytes We focused on the VLRB portion of the humoral response in this analysis because it is the prevalent component of the VLR-based immune system in the sea lamprey1,5. Humoral agglutinin, hemolysin and bactericidal responses after immunization of lamprey and hagfish with heterologous erythrocytes and bacteria have been 1Office of Fundamental Immunology Research, 2Department of Microbiology, 3Department of Pathology and 4Department of Pediatrics, University of Alabama at Birmingham, Birmingham, Alabama, 35294, USA. Correspondence should be addressed to M.D.C. ([email protected]). Received 24 September 2007; accepted 9 January 2008; published online 3 February 2008; doi:10.1038/ni1562 NATURE IMMUNOLOGY VOLUME 9 NUMBER 3 MARCH 2008 319 1 3 1 Plasma treatment: Erythrocyte immunization doses e 5 N on e A ad nti so V L rb R tio B n ad Co so nt rb rol tio n 2 M R ous BC e 7 2 × 10 10 × × 10 6 5 0 2 28 2 7 14 21 Time after immunization (d) 3 <50 <50 <20 0 f Plasma adsorption Figure 1 Production of VLRB antibodies after immunization with heterologous erythrocytes. (a) Time course of the production of H– H+ Anti-VLRB None cells cells beads hemagglutinin titers (small horizontal bars indicate mean) after one Unimmunized lamprey plasma (kDa) immunization (filled circles) or two immunizations (open triangles) at 100 VLRs in 75 immunized lamprey plasma 7 day 14 (arrow) with 1 10 human blood group O erythrocytes; the Mouse 50 mean hemagglutinin titer is significantly higher for ‘boosted’ lampreys mAb to H (n ¼ 3; P o 0.01). (b) Hemagglutinin responses of lampreys 35 immunized twice with various numbers of erythrocytes (mean + s.e.m.; 25 n ¼ 3 lampreys per antigen dose). (c–f) Responses of lampreys Agglutinin Fluorescence (log10) immunized with 1 107 erythrocytes on days 0 and 14; blood samples 3,840 3,840 0 0 titer: were obtained on day 28 after immunization. (c) Specificity of agglutinin responses in lampreys immunized with mouse erythrocytes (open circles) or human erythrocytes (filled circles). Assay antigens, horizontal axis. RBC, red blood cell. (d) Hemagglutinin titers before and after immune plasma adsorption with beads coated with monoclonal antibody to VLRB or control antibody (mean + s.e.m.; n ¼ 7 lampreys). (e) H-antigen reactivity of plasma samples from naive lampreys (black line) and lampreys immunized with erythrocytes (gray line), analyzed with mouse monoclonal antibody to H (gray filled histogram); staining is shown for CHO cells transfected with a1,2fucosyltransferase expressing H antigen. (f) Depletion of H antigen–specific VLRB antibodies from immune plasma by adsorption with H antigen–bearing CHO cells. Data are representative of one (a–c), two (d) or four (e,f) experiments. Events © 2008 Nature Publishing Group http://www.nature.com/natureimmunology 1 Reciprocal 3 hemagglutinin titer (×10 ) 102 3 d 4 H u R ma BC n 103 c 5 Reciprocal 3 hemagglutinin titer (×10 ) b 4 10 Reciprocal hemagglutinin titer (×103) a Reciprocal hemagglutinin titer ARTICLES reported7–14,16,17. However, descriptions of the responsible humoral factors have been either inconsistent or inconclusive in terms of their molecular size, antigen specificity, relative heat stability, immunoglobulin versus nonimmunoglobulin nature, and other physical characteristics7–12,17. For our analysis of the potential function of VLR antibodies in the erythrocyte agglutinin response, we intraperitoneally immunized 2- to 4-year-old sea lamprey larvae approximately 13 cm in length18 with either mouse or human erythrocytes. We used two monoclonal antibodies specific for the VLRB stalk region, 4C4 (immunoglobulin G2b (IgG2b) isotype) and 6C3 (IgM isotype), to measure VLRB antibody responses. After an intraperitoneal injection of 1 107 human erythrocytes, hemagglutin responses peaked about 19 d later. Booster immunization with the same immunogen dosage on day 14 resulted in a VLRB response about 20-fold higher than that of lampreys immunized with a single dose (Fig. 1a). In other experiments, we found that the hemagglutinin response to erythrocytes was dependent on antigen dose (Fig. 1b) and was specific for donor mouse or human erythrocyte antigens (Fig. 1c). To determine whether the erythrocyte agglutination was mediated by VLRB antibodies, we used Sepharose beads coated with antibody to VLRB (anti-VLRB) to remove VLRB antibodies from immune plasma samples. Adsorption with the anti-VLRB-coated beads resulted in almost complete removal of the hemagglutinin activity, whereas adsorption with beads coated with a control antibody of irrelevant specificity had no demonstrable effect (Fig. 1d). These findings indicate that the erythrocyte-specific agglutinins made by immunized lamprey are mainly VLRB antibodies. Erythrocyte carbohydrate specificity of VLRB antibodies Earlier studies of lampreys suggested that agglutinins to blood group O erythrocytes are specific for the H-trisaccharide cell surface antigen that defines this blood type in humans8,11. To assess the H-antigen specificity of the VLRB antibodies, we used Chinese hamster ovary 320 (CHO) cells stably transfected with the a1,2-fucosyltransferase enzyme that generates the H-trisaccharide antigenic determinant19. In these studies, lampreys immunized with human blood group O erythrocytes produced VLRB antibodies that recognized CHO cells expressing the H-trisaccharide antigen (Fig. 1e), whereas they did not produce VLRB antibodies to control CHO cells transfected with vector alone. Adsorption of the immune plasma samples with H antigen–positive cells removed the agglutinating VLR antibodies without noticeably affecting the concentration of VLRB antibodies in the bloodstream (Fig. 1f). These findings confirm that the H trisaccharide is a dominant antigenic determinant in the lamprey humoral response to blood group O erythrocytes, indicate the response is attributable mainly to the production of VLRB antibodies and demonstrate that these antigen-specific VLRB antibodies constitute a minor fraction of the total pool of circulating VLRB antibodies. VLRB antibodies are disulfide-linked multimers The ability of lamprey VLRB antibodies to agglutinate erythrocytes indicated that they were multivalent. Immunoblot analysis of plasma samples to test that possibility showed that VLR antibodies in the circulation were large proteins of over 250 kilodaltons (Fig. 2), whereas the molecular masses predicted for the VLRB proteins on the basis of amino acid composition vary on average between 22 and 30 kilodaltons. Use of 2-mercaptoethanol in sufficient concentration to reduce disulfide bonds resolved the large VLRB antibodies into individual protein components that migrated slightly faster in sizing gels than expected for the predicted molecular masses of VLRB monomers. Many potential O-linked glycosylation sites in the stalk region of the VLRB proteins could account for the difference in predicted protein mass versus that estimated by gel migration. Additionally, we noted a dimeric subcomponent in the form of protein bands of about 70 kilodaltons after treatment with intermediate concentrations of the reducing agent. This analysis suggests VOLUME 9 NUMBER 3 MARCH 2008 NATURE IMMUNOLOGY ARTICLES (kDa) 225 – 150 – 100 – 75 – 35 – 25 – 2-ME concentration: Figure 2 VLRB antibody composition. Immunoblot of lamprey plasma treated with increasing concentrations (wedge) of the reducing agent 2-mercaptoethanol (2-ME), analyzed with monoclonal antibody 4C4 (anti-VLRB). Data are representative of four experiments. that the VLRB antibodies are composed of multiple VLRB monomers linked by disulfide bonds to form large oligomeric molecules composed of dimeric subunits. VLRB antibody response to B. anthracis Studies have indicated that after immunization with B. anthracis exosporium, lamprey produce VLR antibodies to the spore surface glycoprotein BclA2. We reexamined that response to define the antigen dose requirements, kinetics and epitope specificity of the VLRB antibody response. After a single intraperitoneal immunization with 10 mg B. anthracis exosporium, VLRB antibodies to spores were detectable around 7 d later, and peak titers were reached at about 26 d. There were much higher titers of specific VLRB antibodies when lampreys were given a second immunization on day 14 and plasma samples were analyzed on day 28 (Fig. 3a). As noted for the erythrocyte response, increasing the exosporium dose resulted in the production of higher titers of VLRB antibodies to BclA (Fig. 3b). A large portion of the VLRB antibody response seemed to be directed against BclA, as we detected only minimal VLRB reactivity for B. anthracis spores deficient in BclA (Fig. 3c). Unresponsiveness to a soluble protein antigen To determine whether sea lamprey larvae make antigen-specific VLRB antibodies when immunized with a protein antigen, we immunized lamprey with 10 mg of bovine serum albumin (BSA) that was either unmodified, alum precipitated or combined with the commercially available adjuvants Ribi and TiterMax, which contain bacterial products in an emulsion of water in oil. In other immunizations, we used BSA conjugated to the surface of polystyrene beads and injected 1 108 beads either alone or together with 1 mg each of lipopolysaccharide, lipoteichoic acid or peptidoglycan. For these experiments we used an immunization protocol that resulted in a strong VLRB humoral response to B. anthracis exosporium proteins. In this procedure, primary immunization was followed by booster immunization 2 weeks later, and plasma was collected for antibody assessment by enzyme-linked immunosorbent assay (ELISA) at 4 weeks. However, none of these methods of BSA immunization resulted in the production of VLRB antibodies by immunized lamprey that were detectable by ELISA (n ¼ 30 lamprey with four to five per immunization group; data not shown). When we immunized other groups of lampreys with 50 mg keyhole limpet hemocyanin (KLH; n ¼ 10 lamprey), we did not detect VLRB antibodies to this soluble protein antigen by ELISA over a 56-day period. Moreover, lamprey immunized with BSA or KLH did not respond with the lymphoblastoid transformation of circulating Figure 3 VLRB antibody response to * immunization with B. anthracis. (a) ELISA of 2.5 plasma VLRB antibody titers after primary 2.0 2.0 immunization with 10 mg B. anthracis 2.0 exosporium (filled circles) or booster 1.0 1.0 immunization (filled bar) on day 14 (arrow); error bars indicate s.e.m. for n ¼ 3–11 lampreys, 1.5 0 0 except day 49, for which the range of values is BclA-CTD GST 101 102 103 104 control Reciprocal titer for two lampreys. (b) Antigen dose requirement. ELISA of VLRB antibody titers to BclA protein ** 1.0 before () and after intraperitoneal injection of 0.05 mg (m) or 0.5 mg (J) or 5 mg (~) of 1.0 anthrax exosporium on days 0 and 14, measured 0.5 on day 28 (n ¼ 3 lampreys per antigen dose). 0.5 (c) ELISA of the specificity of VLRB antibodies 0.1 for B. anthracis after two immunizations with ** * 0 7 14 21 28 49 5 mg exosporium (mean + s.e.m.; n ¼ 4 Time after immunization (d) 0 lampreys). BclA D, BclA-deficient. *, P o 0.05; B. anthracis B. anthracis B. cereus B. thuringiensis (wild-type) (BclA ∆) **, P o 0.01. (d) ELISA of the VLRB antibody response to BclA-CTD and glutathione S-transferase (GST) control protein (mean + s.e.m.; n ¼ 4 lampreys). *, P o 0.01. Plasma samples in c,d are from immunized lampreys (filled bars) and unimmunized lampreys (open bars). A405, absorbance at 405 nm. Data are representative of one (a,b) or two (c,d) experiments. d A405 b A405 a c A405 A405 © 2008 Nature Publishing Group http://www.nature.com/natureimmunology 50 – As mice make antibodies directed mainly against the carboxyterminal domain of BclA (BclA-CTD) after B. anthracis immunization15,20, we evaluated the lamprey response to this determinant. Our results showed that immunized lamprey also made VLRB antibodies to BclA-CTD (Fig. 3d). Adsorption of immune plasma with BclACTD-coated beads removed most of the reactivity of VLR antibody with B. anthracis spores (data not shown). Moreover, the VLRB antibody response was directed mainly against the B. anthracis strain; we noted much lower titers of VLRB antibody reactivity to spores of the closely related species Bacillus thuringiensis and Bacillus cereus (Fig. 3c). Notably, BclA-CTD of B. cereus differs from BclA-CTD of B. anthracis by approximately 10% of constituent amino acids. These observations indicate that the lamprey VLRB response to B. anthracis exosporium is dose dependent and highly antigen specific. Our results also suggest that the CTD of the BclA surface protein is a chief antigenic determinant for this humoral response. NATURE IMMUNOLOGY VOLUME 9 NUMBER 3 MARCH 2008 321 ARTICLES the abundance of VLRB antibodies in plasma samples, prominent extracellular VLRB staining was evident in the blood vessels and typhlosole sinuses. Conversely, there was H&E minimal VLRB staining in extravascular compartments throughout the lamprey larvae. VLRB+ We also did immunofluorescence analysis of VLRB-bearing cells with cell suspensions freshly prepared from blood, kidney and AntiVLRB typhlosole. Among cells with the light-scatter characteristics of lymphocytes, 15–35% of VLRB– blood cells were surface VLRB+, versus d 50 about 50% in kidney cell suspensions and Blood Kidney Typhlosole b 40 15–30% of the typhlosole cells (Fig. 4b). 31% 18% 47% VLRB-bearing cells from blood and kidney 30 expressed much more VLRB than did those 20 from typhlosole, which also showed greater 10 variability in cell surface VLRB expression 0 (Supplementary Fig. 2 online). Notably, Anti-VLRB fluorescence (log 10) VLRB+ VLRB– there was a temporary loss of VLRB+ cells Figure 4 Tissue distribution of VLRB+ lymphocytes. (a) Immunohistochemical analysis of VLRB+ in the typhlosole but not of VLRB+ cells in cells in paraffin sections stained with hematoxylin and eosin (H&E; top) or with monoclonal kidney or blood immediately after the antibody 6C3 (Anti-VLRB) and DAB as a chromogen marker (bottom). Original magnification, 600. shipment of lamprey larvae by air freight, (b) Immunofluorescence analysis of VLRB surface expression by lymphocytes from blood, kidney and and we recapitulated this phenomenon by typhlosole, for live cells with lymphocyte-like light-scatter characteristics. Numbers above bracketed treatment with exogenous corticosteroid lines indicate percent VLRB+ cells. (c) Transmission electron microscopy of sorted VLRB+ and VLRB– blood cells in the ‘lymphocyte gate’, showing a resting VLRB+ lymphocyte (top) and a thrombocyte with (Supplementary Fig. 3 online). In both characteristic nuclear cleft (bottom). Scale bars, 1 mm. (d) Quantitative PCR analysis of VLR transcripts cases, recovery of the VLRB-bearing popula(relative to glyceraldehyde phosphate dehydrogenase) expressed by sorted VLRB+ and VLRB– cells with tion in the typhlosole occurred over the lymphocyte-like light-scatter characteristics. VLRB– cells represent a mixture of thrombocytes (about ensuing 2–3 weeks. These observations indi85%) and lymphocytes (about 15%). Data are representative of ten (a), 22 (b), two (c) or three (d) cated that mature VLRB+ cells and their experiments (error bars, s.e.m.). soluble VLRB products were confined mainly to the vascular compartment, except for the lymphocytes noted after ‘hyperimmunization’ of lamprey with over typhlosole and the kidney, in which interstitial VLRB+ lymphocytes 25 mg exosporium (discussed below). These model protein immuno- were abundant around the tubules. They also suggested that along gens thus failed to elicit a VLRB antibody response, even when given with other blood cell types21, the VLRB-producing cells may be with adjuvants, in aggregated form or coated onto the surface of a generated in the larval typhlosole. solid matrix, in the case of BSA. VLR expression profile of VLRB+ lymphocytes Tissue distribution of VLRB+ lymphocytes When we isolated VLRB+ and VLRB– cells in the ‘lymphocyte gate’ by To determine the cellular basis of the lamprey humoral response to a fluorescence-activated cell sorting and analyzed them by transmission particulate antigen, we examined the tissue distribution of VLRB+ cells electron microscopy, we found that the VLRB+ cells in unimmunized by immunohistochemical staining with the two monoclonal anti- lamprey resembled the small lymphocytes of jawed vertebrates bodies (4C4 and 6C3) to the invariant VLRB stalk region. In pilot (Fig. 4c). VLRB+ lymphocytes typically have a relatively large nucleus studies, 6C3 yielded better definition of the VLRB+ lymphocytes, as with peripheral concentration of the chromatin. The narrow rim of paraffin-embedded sections had less background staining (specificity cytoplasm surrounding the nucleus contains relatively few distinguishof 6C3 for VLRB, Supplementary Fig. 1 online). Therefore, we used able organelles, such as mitochondria. Many of the VLRB– cells in the 6C3 for tissue analysis, which showed the presence of VLRB+ cells in ‘lymphocyte gate’ were identifiable as thrombocytes, with a characterthe kidney and typhlosole as well as in blood vessels throughout the istic deep nuclear cleft and relatively abundant cytoplasm (Fig. 4c, lamprey larvae. Notably, we did not detect VLRB+ lymphocytes in or bottom), whereas cells with lymphocyte morphology were in the beneath the epithelium of the intestine, which in the filter-feeding minority (about 15%). Analysis of VLR transcripts in these isolated larval stage is essentially an unvariegated tube extending from the last VLRB+ and VLRB– populations of cells indicated that the purified gill slit region to the cloaca. Over most of its length, the intestine is VLRB+ cells expressed exclusively VLRB transcripts, whereas cells of folded like an elongated horseshoe over the typhlosole, which is filled the VLRB– population expressed VLRA transcripts but did not express with hematopoietic lineage cells surrounding blood-filled sinuses. VLRB transcripts (Fig. 4d). We conclude from these results that VLRB+ lymphocytes were dispersed throughout the typhlosole, in VLRB+ and VLRA+ cells belong to separate lymphocyte populations. which they showed greater morphological diversity and variability in VLR staining intensity than did VLRB+ lymphocytes elsewhere The VLRB+ lymphocyte response to immunization (Fig. 4a). Small VLRB+ cells were intermixed with cells of other Intraperitoneal injection of lamprey with a ‘cocktail’ of antigens and hematopoietic lineages in ventral interstitial regions of the kidneys phytomitogens has been shown to induce a lymphoblastoid response1, surrounding the renal tubules. The blood vessels in the gill regions and and we noted a similar response after injecting a large dose of elsewhere contained many VLRB+ lymphocytes. In accordance with B. anthracis exosporium (over 25 mg; Supplementary Fig. 4 online). 322 Kidney Typhlosole c Relative transcript expression Gill Events © 2008 Nature Publishing Group http://www.nature.com/natureimmunology a VOLUME 9 NUMBER 3 MARCH 2008 NATURE IMMUNOLOGY ARTICLES b * 6.0 B. anthracis binding Unimmunized Antigen-binding + VLRB cells (%) a 4.0 2.0 Figure 5 Antigen-binding VLRB+ cells before and after immunization with B. anthracis. (a) Percent B. anthracis spore–binding VLRB+ cells before (Naive) and 28 d after (Immunized) immunization with 10 mg B. anthracis exosporium on days 0 and 14 (error bars indicate s.e.m. for n ¼ 3 lampreys per group). *, P o 0.05. (b) Flow cytometry of antigen-binding VLRB+ cells in blood samples from naive and immunized lampreys analyzed by costaining with monoclonal antibody 4C4 and fluorescence-tagged spores. Numbers above bracketed lines indicate percent cells that bound spores. (c) Binding of fluorochrome-labeled B. anthracis spores or S. typhimurium by VLRB+ lymphocytes from lampreys immunized with B. anthracis exosporium and S. typhimurium. Numbers in quadrants indicate percent cells binding only B. anthracis (top left), only S. typhimurium (bottom right) or both (top right). (d) Time-course analysis of antigen-binding VLRB+ cells in blood after a single injection of B. anthracis exosporium (10 mg), analyzed by costaining with monoclonal antibody 4C4 and fluorochrome-tagged spores; error bars indicate s.e.m. for n ¼ 3–9 lampreys per data point, except at 49 d, for which mean and range are shown for two lampreys. Data are representative of one (a,d), four (b) or three (c) experiments. B. cereus binding 1% 1% 7% 1% 90° light scatter c Spore-binding VLRB+ cells 1.60 0.01 0.40 d 3.0 Spore-binding VLRB+ cells (%) VLRB+ Forward scatter 1.5 0 0 S. typhimurium binding 7 14 21 Time after immunization (d) Most of these responding lymphoblastoid cells had much lower cell surface expression of VLRB (Supplementary Fig. 4). These observations suggested that when given in a sufficient dose, the B. anthracis exosporium serves as a lymphocyte mitogen in the lamprey. These results also raised the issue of whether a global mitogen response is required for the VLR antibody response to the BclA antigen. When we immunized lamprey larvae with lower doses of exosporium, however, antigen-specific VLRB antibodies were produced in the absence of a lymphoblastoid response that we could discern by flow cytometry (data not shown). To examine the response of antigen-specific VLRBbearing cells, we determined the frequency of B. anthracis spore– binding VLRB+ cells before and after two immunizations with exosporium (10 mg). When we examined blood samples on day 28, we noted a fourfold higher percentage of VLRB+ cells that bound fluorescence-labeled B. anthracis spores, whereas background numbers of B. cereus spore–binding cells were unchanged (Fig. 5a,b). As the larger numbers of antigen-binding VLRB+ cells could have reflected binding by cytophilic VLRB antibodies, we incubated blood cells from naive lamprey in immune plasma samples containing high titers of anthrax-specific VLRB antibodies. When we examined the preincubated cells for antigen binding, we noted no greater numbers of anthrax spore–binding cells (Supplementary Fig. 5 online). As Figure 6 Proliferation of VLRB+ cells in lampreys immunized with B. anthracis exosporium. (a–d) Immunofluorescence analysis of BrdU incorporation by VLRB+ cells in lampreys before (a,c) and after (b,d) they were immunized with a single injection of B. anthracis exosporium (25 mg), followed 5 d later by a pulse for 7 h with BrdU before processing for tissue immunohistochemical analysis. Sections of gills (a,b) and ventral kidney region (c,d) are stained with the nuclear dye DAPI (4,6-diamidino-2phenylindole; blue) and for BrdU (red) and VLRB (green). White arrowheads indicate BrdU-incorporating VLRB+ cells in the gills (b) and kidneys (d) of immunized lampreys. *, representative plasmacytoid cell. Original magnification, 10. (e) Percent BrdU+ VLRB+ cells in tissues before (open bars) and after (filled bars) immunization as described in a–d (error bars indicate s.e.m. for n ¼ 3 lampreys per group). *, P o 0.05. Data are representative of three experiments. NATURE IMMUNOLOGY VOLUME 9 NUMBER 3 MARCH 2008 28 49 another test of the specificity of the antigen-binding VLRB+ lymphocytes, we immunized larvae simultaneously with two immunogens, B. anthracis exosporium and heat-killed Salmonella typhimurium, then determined whether this dual immunization resulted in the appearance of lymphocytes that could bind both antigens. We found discrete subpopulations of cells that bound one immunogen or the other, a b c d Gills Kidney e 10 BrdU+ (%) B. anthracis binding © 2008 Nature Publishing Group http://www.nature.com/natureimmunology Naive lmmunized Immunized with B. anthracis exosporium Events 0.0 * * Gills Kidney 5 0 Typhlosole 323 ARTICLES a © 2008 Nature Publishing Group http://www.nature.com/natureimmunology 90° light scatter VLRB+ 0 0 VLRB– + VLRB VLRB+ VLRB– VLRB– 169 0 0 1 Forward scatter b which suggested that VLRB+ cells bound antigen via their own endogenous antigen receptor and not via cytophilic antigen receptors (Fig. 5c). When we measured the percentage of VLRB+ cells that bound to fluorescence-labeled spores over a 7-week interval after a single injection of exosporium (10 mg), we found the highest percentage of spore-binding VLRB+ cells on about day 26 (Fig. 5d). Given these results, the increase in numbers of antigen-binding VLRB+ cells after B. anthracis exosporium immunization is most easily explained by antigen-induced cellular proliferation. To test that interpretation, we immunized lampreys with exosporium (25 mg) and pulsed them with 5-bromodeoxyuridine (BrdU) before looking for VLRB+ cells undergoing proliferation. When we examined the lymphoid tissues later by immunohistology (Fig. 6), we found many more BrdUcontaining VLRB+ cells in immunized lampreys than in naive BrdUtreated lampreys. The greater numbers of BrdU-containing VLRB+ cells were much higher in the gills and kidney than in the typhlosole (Fig. 6e). We also noted VLRB-containing cells with lymphoblastoid and plasmacytoid features more frequently in the gill region and kidneys than in the typhlosole of immunized larvae (Fig. 6). Plasmacytoid cells secrete VLRB antibodies To characterize the cells that secreted the VLRB antibodies after antigenic stimulation, we isolated VLRB+ and VLRB– subpopulations of cells from immunized lamprey on the basis of their relative cell size and analyzed by enzyme-linked immunospot (ELISPOT) assay their ability to secrete VLRB antibodies to BclA-CTD. We placed subpopulations of cells derived from blood, kidney and typhlosole tissue in culture for 18 h before analyzing VLRB antibody secretion. In these experiments, we found cells that secreted BclA-CTD-specific antibodies exclusively among the relatively large VLRB-bearing cells (Fig. 7a). When we isolated these VLRB-producing cells for morphological evaluation by transmission electron microscopy, we found that they were large plasmacytoid cells with copious cytoplasm containing many organelles and a prominent network of rough endoplasmic reticulum (Fig. 7b). The VLRB-antibody secreting cells were more abundant in blood and kidney samples than in the typhlosole at 2 weeks after booster immunization with B. anthracis exosporium. The frequency of cells that secreted antigen-specific VLRB antibodies 324 Figure 7 Characterization of VLRB-secreting cells induced by immunization with B. anthracis exosporium. Analysis of cells from the kidneys of lamprey larvae (n ¼ 6) immunized with B. anthracis exosporium and given booster immunization with 5 mg exosporium on day 14; cells were pooled for analysis 14 d later. (a) ELISPOT of the secretion of antigen-specific VLRB antibodies by VLRB+ and VLRB– cells sorted into three populations (circled in dot plot) on the basis of light-scatter characteristics. Numbers beside ELISPOT plate images indicate cell counts. (b) Transmission electron microscopy of large VLRB+ antibody-producing cells showing plasmacyte morphology with expanded rough endoplasmic reticulum; right, enlargement of a representative portion of the main image at left. Original magnification, 36,000. Data are representative of three (a) or two (b) experiments. (per 1 106 leukocytes) was 215 ± 101 for blood, 272 ± 100 for the kidney and 93 ± 25 in the typhlosole (mean ± s.e.m.). The observations reported above indicate that immunization of lamprey with an effective immunogen induces antigen-specific lymphocytes to undergo lymphoblastoid transformation, proliferation and differentiation into plasmacytes that secrete antigen-specific VLRB antibodies while continuing to express cell surface VLRB antibodies. DISCUSSION Our analysis here has indicated that VLRB-bearing cells belong to a distinct lineage of lymphocytes that dominate the humoral response of lamprey to antigenic stimulation. The prototypic small VLRB+ cells constitute a chief lymphocyte subpopulation in the hematopoietic typhlosole, circulation and ventral regions of the kidney in healthy lampreys. Lamprey VLRB lymphocytes, unlike T lymphocytes and B lymphocytes in jawed vertebrates, are not found in organized lymphoid organs, such as lymph nodes, spleen or intestinal lymphoid tissues. Follicular accumulation of VLRB+ lymphocytes was not demonstrable in 3-year-old lamprey even after ‘hyperimmunization’ and, more unexpectedly, VLRB-bearing lymphocytes were not present in or beneath the intestinal epithelium. A convincing thymus-like structure has not been found in lamprey22,23, and we did not find accumulation of VLRB+ lymphocytes in the gill regions. As for VLRA-producing lymphocytes, it is notable that we found VLRA transcripts only in the mixed VLRB– population. That finding suggested that the VLRA and VLRB loci, which in hagfish are far apart on the same chromosome24, are under different regulation. When VLRA-specific antibodies become available to allow direct identification of VLRA-expressing cells, it will be useful to determine their contribution to the immune response and their relationship to the VLRB lymphocytes. Our analysis of the requirements for the induction of humoral responses in lamprey indicated that particulate antigens initiated strong VLRB antibody responses, whereas soluble antigens did not. We failed to detect production of VLRB antibodies to the model protein antigens BSA and KLH over an 8-week period after immunization, regardless of the mode of immunization. Concordant results have been obtained in other studies that failed to demonstrate agglutinin responses with antigen-coated erythrocytes after immunization with BSA, KLH or bovine g-globulin7,9. In contrast, VLRB antibodies to the repetitive protein and carbohydrate epitopes on the surface of the representative particulate antigens B. anthracis exosporium and mammalian erythrocytes were consistently induced. Other studies have noted agglutinin production after immunization with Brucella abortus7, and in unpublished studies, we have found that lamprey larvae make VLRB antibodies to other particulate immunogens bearing repetitive cell surface antigens, including Escherichia coli, S. typhimurium, influenza virus and human lymphocytes. This pattern of antigen responsiveness is reminiscent of the mammalian antibody VOLUME 9 NUMBER 3 MARCH 2008 NATURE IMMUNOLOGY © 2008 Nature Publishing Group http://www.nature.com/natureimmunology ARTICLES responses to T cell–independent antigens and is in agreement with the apparent absence of a thymus-dependent T lymphocyte population in lamprey. The mechanisms by which particulate antigens initiate specific VLRB responses are unknown. VLRB molecules are attached to the lymphocyte surface by glycosylphosphatidylinositol linkage1 and therefore lack a cytosolic portion that could trigger intracellular signaling pathways of lymphocyte activation recognized at present. Although ligation of some glycosylphosphatidylinositol-linked molecules such as Thy-1, CD16b and CD14 may trigger cellular activation, the mechanisms of signal induction are either unclear or involve interaction with transmembrane adaptor proteins that have signaling capacity25–27. The latter mode could be operative in VLRB lymphocytes, or the glycosylphosphatidylinositol-linked VLRB molecules could serve merely as antigen-focusing receptors to facilitate contact of the immunogen with a secondary mitogenic receptor, such as a Toll-like receptor or other pattern-recognition molecule with signaling capacity. Two genes encoding Toll-like receptors have been identified in lamprey28. However, we found that immunization with polystyrene beads coated with BSA failed to elicit an immune response, even when given together with candidate Toll-like receptor ligands. Although the cellular activation mechanism is still unknown, our studies indicate that cells bearing antigen-specific VLRB receptors are activated by immunization to undergo lymphoblastoid transformation, proliferation and differentiation into mature VLRB antibody–secreting cells. Moreover, over 20fold higher VLRB antibody titers were induced by booster immunization with human blood group O erythrocytes, and we found that this response was directed specifically against the trisaccharide H antigen. The features of this response are most easily explained by clonal expansion of the antigen-specific VLRB lymphocytes, although formal proof of this interpretation is needed. VLRB-bearing lymphocytes are thus very similar to mammalian B lymphocytes in their response to immune stimulation, in keeping with the idea that cells with lymphocyte-like characteristics evolved in a common ancestor of lamprey and jawed vertebrates29,30. That leads us to speculate that the immunoglobulin-based variable-(diversity)joining rearrangement system for the generation of B lymphocytes may have evolved in an early ancestor of jawed vertebrates, perhaps one of the ostracoderms, before the evolution of thymus-derived T lymphocytes and major histocompatibility complexes for antigen presentation. However, this hypothesis will probably remain untested, because key representatives in the vertebrate evolutionary pathway are now extinct31. The results of our analysis of the antigen-induced responses of VLRB+ lymphocytes are consistent with observations reported in earlier studies of the immune response in agnathans. In both lamprey and hagfish, immunization with bacteria or heterologous erythrocytes elicits a humoral response that can be measured by antigen agglutination assay7–14. Moreover, hemagglutinins to human O+ erythrocytes seem to be specific for the polysaccharide H antigen8,11. Erythrocytebinding cells increase in frequency within days of erythrocyte immunization32, and cells with the morphological features of plasma cells have also been reported33–36. All of those observations may now be explained by the antigen recognition and responsiveness of a diverse repertoire of VLRB-expressing lymphocytes that differentiate into plasmacytes that secrete multimeric VLRB antibodies into the circulation. Our analysis suggests that such antibodies are large multimeric molecules composed of disulfide-linked pairs of identical VLRB chains, a structural configuration that is supported by analysis of recombinant VLRB antibodies37. The multivalency of VLRB antibodies accounts for their ability to agglutinate particulate NATURE IMMUNOLOGY VOLUME 9 NUMBER 3 MARCH 2008 immunogens with repetitive antigenic epitopes. Further studies are needed to elucidate the molecular mechanisms that allow lamprey plasmacytes to simultaneously express cell surface VLRB and secrete multimeric VLR antibodies. Finally, our observations offer clues about the primary generation site for VLRB lymphocytes in lamprey larvae. We noted relatively low and more variable VLRB expression before and after immunization for the population of lymphocytes in the typhlosole relative to the lymphocyte populations in blood and kidney. Notably, animal stress and treatment with exogenous corticosteroids led to loss of VLRbearing cells exclusively in the typhlosole. These findings are reminiscent of the corticosteroid susceptibility of immature B lineage cells in the avian bursa of Fabricius38 and the mammalian bone marrow39 and of immature mammalian thymocytes40. In addition to their implied immaturity, relatively few typhlosole-based VLRB lymphocytes responded to immunization by proliferating and differentiating into antibody-secreting plasmacytes. These findings collectively indicate that VLRB+ lymphocytes are generated along with other types of blood cells in the typhlosole. METHODS Animal maintenance and immunization. Sea lamprey larvae (11–15 cm in length and 2–4 years of age; Lamprey Services) were from the Great Lakes of North America. These outbred larvae are more homogeneous than lamprey from the sea, presumably because of founder influence41. Lamprey were maintained in sand-lined aquariums at 16–18 1C and were fed brewer’s yeast. Purified exosporium of the Sterne strain of B. anthracis15, erythrocytes or recombinant proteins were injected intraperitoneally into lamprey anesthetized by immersion in ethyl 3-aminobenzoate methanesulfonic acid (0.1 g/l; Sigma). Monoclonal antibodies to VLR and recombinant VLR antibody. Two mouse monoclonal antibodies were produced by ‘hyperimmunization’ of mice with a recombinant VLRB invariant stalk–region protein produced in E. coli and subsequent fusion of regional lymph node cells with the nonproductive Ag8.653 myeloma variant42. Two hybridoma clones that produced antibodies with VLRB specificity, 6C3 (IgM) and 4C4 (IgG2b), were identified by ELISA and flow cytometry screening2 (Supplementary Fig. 1). By immunofluorescence staining of viable cells and by immunohistochemical staining of fixed sections, 6C3 and 4C4 were shown to recognize the same lymphocyte populations in lamprey blood and tissues; 4C4 was also shown to be reactive with VLRB protein by immunoblot analysis. Immunohistochemistry, immunofluorescence and electron microscopy. Lamprey were killed by immersion in ethyl 3-aminobenzoate methanesulfonic acid (1 g/l) so tissue and blood samples could be obtained. For immunohistology, corpse transections 1 cm in length were fixed in 10% (vol/vol) neutral buffered formalin and were embedded in paraffin. Cut sections were deparaffinized and rehydrated by sequential emersion in 100%, 95% and 70% (vol/vol) ethanol before antigen retrieval by heating of the sections for 10 min at 15 p.s.i. in 0.01 M citric acid, pH 6 (for 6C3), or in 0.01 M EDTA, pH 8 (for 4C4)43. Sections were then treated for 5 min with 3% (vol/vol) hydrogen peroxide before being blocked for 30 min with 3% (vol/vol) goat serum. Processed tissue sections were incubated for 1 h at 22 1C with one of the primary antibodies before being washed with Tris-buffered saline, pH 7.6, followed by the addition, for 20 min each, of biotinylated secondary antibody and streptavidin–horseradish peroxidase (SIG-32252; Signet Laboratories), then the addition of the diaminobenzidine substrate (BioGenex) for chromogenic labeling. Labeled slides were immersed briefly in Mayer’s hematoxylin for counterstaining, then were dehydrated in sequential baths of ethanol and xylene before coverslips were applied. The same protocol was used for immunofluorescence, except that coverslips were placed after addition of the secondary antibody with Prolong Gold and 4,6-diamidino-2-phenylindole mounting media (Invitrogen). For electron microscopy, sorted blood cells were resuspended for 4 h at 4 1C in sodium cacodylate or Sorsenson’s buffer with 2.5% (vol/vol) glutaraldehyde. Cells were then post-fixed for 1 h in 1% (wt/vol) 325 ARTICLES © 2008 Nature Publishing Group http://www.nature.com/natureimmunology osmium tetroxide, were dehydrated in a graded series of acetone and were embedded in epoxy resin. For quantification of lymphocytes in the VLRB+ and VLRB– populations of cells with lymphocyte-like light-scatter characteristics, cytospins of sorted cells were fixed for 30 s in methanol and then were stained for 2 min with Wright-Geimsa stain (EM Science). Stained slides were washed in water and coverslips were added before analysis by light microscopy. Detection of BrdU+VLRB+ cells by immunohistochemistry. Lamprey were immunized with 25 mg B. anthracis exosporium and were immediately returned to their water tanks. Then, 5 d later, 200 mg BrdU (Beckton Dickinson) was injected in 60 ml of 0.667% (vol/vol) PBS and lamprey were returned to their tanks for 7 h before being killed, followed by tissue processing and embedding of tissues in paraffin. Monoclonal antibody 6C3 (anti-VLRB; IgM) and antiBrdU (Bu20a, IgG1; M0744; Dako) plus isotype-specific fluorescence-labeled secondary antibodies (tetramethylrhodamine isothiocyanate–labeled goat anti– mouse IgG (1030-03) and fluorescein isothiocyanate–labeled goat anti–mouse IgM (1021-02); Southern Biotech) were used for fluorescence staining as described above. Antigens and VLR antibody assays. BSA immunization consisted of injection of 10 mg BSA in 50 ml of one of the following vehicles: sterile 0.66% (vol/vol) PBS; 200 mg Al(OH)3 absorbed with protein for 4 h before injection; or emulsion with Ribi or TiterMax Gold adjuvant (Sigma) prepared according to the manufacturer’s protocol. For BSA-coated beads, BSA was conjugated to carboxylate polystyrene beads 1 mm in diameter with a carbodiimide kit according to the manufacturer’s protocol (Polysciences), and lipopolysaccharide, lipoteichoic acid and peptidoglycan (Invivogen) were added before injection. Erythrocytes from C57BL/6 mice or human donors of blood group O were washed three times before injection. For antibody assays, washed erythrocytes (5 106) were mixed with lamprey plasma at various dilutions and were allowed to settle for 1 h in conical-bottomed microwell plates before visual assessment of agglutination after plates were tilted for 2 min at 80 1C. ELISAs were done as described2 in plates coated with BSA, KLH or BclA-CTD at a concentration of 5 mg/ml or B. anthracis spores, BclA-deficient B. anthracis spores, B. cereus strain T spores and B. thuringiensis subspecies kurstaki spores at a density of 1 106 spores per well20. The reactivity of VLR with H antigen was determined by incubation of test plasma samples with CHO cells stably transfected with constructs for a1,2-fucosyltransferase or vector alone19. CHO cells were then stained by incubation for 10 min each with monoclonal antibody 4C4 and phycoerythrin-conjugated goat antibody to mouse immunoglobulin heavy plus light chain (1031-09; Southern Biotech) before analysis of immunofluorescence with a CyAn ADP high-performance flow cytometer (Dako). For plasma VLR adsorption, test samples were mixed for 1 h at 4 1C with 4C4, BclA-CTD conjugated to Sepharose or CHO cells (3 106) fixed with paraformaldehyde. Beads or cells were ‘spun down’ and supernatants were transferred to a fresh test tube, then the adsorption process was repeated before analysis of antigen reactivity by agglutination, ELISA or immunoblot assay. For staining of lamprey lymphocytes with fluorescent spores or bacteria, 1 106 leukocytes were mixed for 20 min on ice with 1 106 spores and/or 5 107 S. typhimurium labeled44 with Alexa Fluor 488 or Alexa Fluor 647 (Invitrogen) and then were incubated for an additional 10 min with 4C4. Cells were then washed and were incubated for 10 min on ice with phycoerythrin-conjugated goat antibody to mouse immunoglobulin (heavy plus light chain; Southern Biotech) before being washed twice and analyzed by flow cytometry. For cytophilic VLR antibody–binding assays, leukocytes from naive lampreys were incubated for 30 min at 22 1C in plasma from ‘hyperimmunized’ lampreys before being washed and then incubated with fluorochrome-labled spores. Dexamethasone treatment of lamprey larvae. Lamprey were injected intraperitoneally with 3–30 mg dexamethasone (Sigma) or were maintained for 3 d in tanks with 250 mg/ml of dexamethasone. Lamprey were killed 2 d later, then leukocytes and organs were collected for flow cytometry. ELISPOT analysis of VLR-secreting cells. Microwells of 96-well plates (Millipore) were coated overnight at 4 1C with 100 ml recombinant BclA-CTD20 (50 mg/ml), then were blocked for 2 h at 37 1C with 1% (vol/vol) BSA in PBS before the addition of test cell suspensions in Iscove’s modified DMEM (Mediatech) supplemented with 10% (vol/vol) FBS, L-glutamine, penicillin, 326 streptomycin, insulin and transferrin, followed by 18 h of incubation at 25 1C in 5% CO2. Cells were then ‘washed away’ with PBS before the addition, for 1 h at 37 1C, of 4C4 (1 mg/ml) in 1% (vol/vol) BSA. After wells were washed with 0.5% (vol/vol) Tween in PBS, horseradish peroxidase–conjugated goat antibody to mouse immunoglobulin (1010-04; Southern Biotech) was added for 1 h at 37 1C before wells were washed with Tween in PBS and then PBS alone. AEC (3-amino-9-ethyl carbazole) peroxidase substrate (Moss) was then added for 1 h before wells were washed with deionized water and VLR antibody spots were counted with Immunospot 2.0 software (Cellular Technology). Immunoblot analysis. Plasma samples (1 ml) were separated by 10% SDSPAGE with or without 0.25%, 0.5% or 5% (vol/vol) 2-mercaptoethanol, then were transferred to a nitrocellulose membrane. Membranes were blocked with 3% (wt/vol) milk, then were incubated for 1 h with 4C4 and were washed five times with 0.5% (vol/vol) Tween in PBS before the addition of horseradish peroxidase–conjugated goat antibody to mouse (Southern Biotech) and a final wash 1 h later. The SuperSignal chemiluminescent kit (Pierce) was used for the detection of VLR-antibody conjugates. Quantitative PCR. RNA was extracted from cells (sorted as VLRB+ and VLRB–) with Trizol (Invitrogen) and RNeasy with on-column DNA digestion (Qiagen) according to the manufacturer’s protocol. First-strand cDNA was generated with random hexamer primers and Superscript III (Invitrogen). Primers designed at splice sites (Supplementary Table 1 online), where known, were used for quantitative PCR with SYBR Green on a 7900HT ABI Prism (Applied Biosystems). Statistical analysis. Statdisk software (version 10.0.0) and a two-sample Student’s t-test were used for statistical analysis. Note: Supplementary information is available on the Nature Immunology website. ACKNOWLEDGMENTS We thank L. Stansell for providing human erythrocytes; L. Millican and E. Weeks for assistance with electron microscopy; and M. Flurry for help with the preparation of figures. Supported by the National Institutes of Health (AI72435 and AI57699). AUTHOR CONTRIBUTIONS M.N.A., B.R.H., W.E.G., C.L.T. and M.D.C. designed the research; M.N.A., B.R.H. and A.S. did the research; C.R.S., L.A.G., G.L.G. and J.A.B. contributed new reagents and/or analytic tools; M.N.A., B.R.H., A.S., G.L.G., W.E.G., C.L.T. and M.D.C. analyzed data; and M.N.A. and M.D.C. wrote the paper. Published online at http://www.nature.com/natureimmunology Reprints and permissions information is available online at http://npg.nature.com/ reprintsandpermissions 1. Pancer, Z. et al. Somatic diversification of variable lymphocyte receptors in the agnathan sea lamprey. Nature 430, 174–180 (2004). 2. Alder, M.N. et al. Diversity and function of adaptive immune receptors in a jawless vertebrate. Science 310, 1970–1973 (2005). 3. Pancer, Z. et al. Variable lymphocyte receptors in hagfish. Proc. Natl. Acad. Sci. USA 102, 9224–9229 (2005). 4. Kim, H.M. et al. Structural diversity of the hagfish variable lymphocyte receptors. J. Biol. Chem. 282, 6726–6732 (2007). 5. Rogozin, I.B. et al. Evolution and diversification of lamprey antigen receptors: evidence for involvement of an AID-APOBEC family cytosine deaminase. Nat. Immunol. 8, 647–656 (2007). 6. Nagawa, F. et al. Antigen-receptor genes of the agnathan lamprey are assembled by a process involving copy choice. Nat. Immunol. 8, 206–213 (2007). 7. Finstad, J. & Good, R.A. The evolution of the immune response. 3. Immunologic responses in the lamprey. J. Exp. Med. 120, 1151–1168 (1964). 8. Boffa, G.A., Fine, J.M., Drilhon, A. & Amouch, P. Immunoglobulins and transferrin in marine lamprey sera. Nature 214, 700–702 (1967). 9. Marchalonis, J.J. & Edelman, G.M. Phylogenetic origins of antibody structure. 3. Antibodies in the primary immune response of the sea lamprey, Petromyzon marinus. J. Exp. Med. 127, 891–914 (1968). 10. Linthicum, D.S. & Hildemann, W.H. Immunologic responses of Pacific hagfish. 3. Serum antibodies to cellular antigens. J. Immunol. 105, 912–918 (1970). 11. Pollara, B., Litman, G.W., Finstad, J., Howell, J. & Good, R.A. The evolution of the immune response. VII. Antibody to human ‘‘O’’ cells and properties of the immunoglobulin in lamprey. J. Immunol. 105, 738–745 (1970). VOLUME 9 NUMBER 3 MARCH 2008 NATURE IMMUNOLOGY © 2008 Nature Publishing Group http://www.nature.com/natureimmunology ARTICLES 12. Litman, G.W., Finstad, F.J., Howell, J., Pollara, B.W. & God, R.A. The evolution of the immune response. 3. Structural studies of the lamprey immuoglobulin. J. Immunol. 105, 1278–1285 (1970). 13. Fujii, T., Nakagawa, H. & Murakawa, S. Immunity in lamprey. I. Production of haemolytic and haemagglutinating antibody to sheep red blood cells in Japanese lampreys. Dev. Comp. Immunol. 3, 441–451 (1979). 14. Hagen, M., Filosa, M.F. & Youson, J.H. The immune response in adult sea lamprey (Petromyzon marinus L.): the effect of temperature. Comp. Biochem. Physiol. A 82, 207–210 (1985). 15. Steichen, C., Chen, P., Kearney, J.F. & Turnbough, C.L., Jr. Identification of the immunodominant protein and other proteins of the Bacillus anthracis exosporium. J. Bacteriol. 185, 1903–1910 (2003). 16. Acton, R.T., Weinheimer, P.F., Hildemann, W.H. & Evans, E.E. Induced bactericidal response in the hagfish. J. Bacteriol. 99, 626–628 (1969). 17. Acton, R.T., Weinheimer, P.F., Hildemann, W.H. & Evans, E.E. Bactericidal antibody response in the Pacific hagfish, Eptatretus stoutii. Infect. Immun. 4, 160–166 (1971). 18. Purvis, H.A. in Technical Report No. 35 1–36 (Great Lakes Fishery Commission, Ann Arbor, 1979). 19. Prieto, P.A. et al. Expression of human H-type a1,2-fucosyltransferase encoding for blood group H(O) antigen in Chinese hamster ovary cells. Evidence for preferential fucosylation and truncation of polylactosamine sequences. J. Biol. Chem. 272, 2089–2097 (1997). 20. Boydston, J.A., Chen, P., Steichen, C.T. & Turnbough, C.L.,, Jr. Orientation within the exosporium and structural stability of the collagen-like glycoprotein BclA of Bacillus anthracis. J. Bacteriol. 187, 5310–5317 (2005). 21. Potter, I.C., Percy, R., Barber, D.L. & Macey, D.J. in The Morphology, Development and Physiology of Blood Cells (eds. Hardisty, M.W. & Potter, I.C.) Ch. 32, 233–286 (Academic, London, 1982). 22. Finstad, J., Papermaster, B.W. & Good, R.A. Evolution of the immune response. II. Morphologic studies on the origin of the thymus and organized lymphoid tissue. Lab. Invest. 13, 490–512 (1964). 23. Boehm, T. & Bleul, C.C. The evolutionary history of lymphoid organs. Nat. Immunol. 8, 131–135 (2007). 24. Kasamatsu, J., Suzuki, T., Ishijima, J., Matsuda, Y. & Kasahara, M. Two variable lymphocyte receptor genes of the inshore hagfish are located far apart on the same chromosome. Immunogenetics 59, 329–331 (2007). 25. Kroczek, R.A., Gunter, K.C., Germain, R.N. & Shevach, E.M. Thy-1 functions as a signal transduction molecule in T lymphocytes and transfected B lymphocytes. Nature 322, 181–184 (1986). 26. Hundt, M. & Schmidt, R.E. The glycosylphosphatidylinositol-linked Fcg receptor III represents the dominant receptor structure for immune complex activation of neutrophils. Eur. J. Immunol. 22, 811–816 (1992). NATURE IMMUNOLOGY VOLUME 9 NUMBER 3 MARCH 2008 27. Stefanova, I. et al. Lipopolysaccharide induces activation of CD14-associated protein tyrosine kinase p53/56lyn. J. Biol. Chem. 268, 20725–20728 (1993). 28. Ishii, A. et al. Lamprey TLRs with properties distinct from those of the variable lymphocyte receptors. J. Immunol. 178, 397–406 (2007). 29. Pancer, Z. & Cooper, M.D. The evolution of adaptive immunity. Annu. Rev. Immunol. 24, 497–518 (2006). 30. Cooper, M.D. & Alder, M.N. The evolution of adaptive immune systems. Cell 124, 815–822 (2006). 31. Forey, P.L. & Janvier, P. Agnathans and the origin of jawed vertebrates. Nature 361, 129–134 (1993). 32. Fujii, T., Nakagawa, H. & Murakawa, S. Immunity in lamprey. II. Antigen-binding responses to sheep erythrocytes and hapten in the ammocoete. Dev. Comp. Immunol. 3, 609–620 (1979). 33. Kilarski, W. & Plytycz, B. The presence of plasma cells in the lamprey (Agnatha). Dev. Comp. Immunol. 5, 361–366 (1981). 34. Zapata, A., Ardavin, C.F., Gomariz, R.P. & Leceta, J. Plasma cells in the ammocoete of Petromyzon marinus. Cell Tissue Res. 221, 203–208 (1981). 35. Fujii, T. Electron microscopy of the leucocytes of the typhlosole in ammocoetes, with special attention to the antibody-producing cells. J. Morphol. 173, 87–100 (1982). 36. Hagen, M., Filosa, M.F. & Youson, J.H. Immunocytochemical localization of antibodyproducing cells in adult lamprey. Immunol. Lett. 6, 87–92 (1983). 37. Herrin, B.R. et al. Structure and specificity of lamprey monoclonal antibodies. Proc. Natl. Acad. Sci. USA, published online 18 January 2008 (doi:10.1073/ pnas.0711619105). 38. Rusu, V.M. & Cooper, M.D. In vivo effects of cortisone on the B cell line in chickens. J. Immunol. 115, 1370–1374 (1975). 39. Igarashi, H. et al. Early lymphoid progenitors in mouse and man are highly sensitive to glucocorticoids. Int. Immunol. 17, 501–511 (2005). 40. Ashwell, J.D., Lu, F.W. & Vacchio, M.S. Glucocorticoids in T cell development and function. Annu. Rev. Immunol. 18, 309–345 (2000). 41. Bryan, M.B. et al. Patterns of invasion and colonization of the sea lamprey (Petromyzon marinus) in North America as revealed by microsatellite genotypes. Mol. Ecol. 14, 3757–3773 (2005). 42. Kearney, J.F., Radbruch, A., Liesegang, B. & Rajewsky, K. A new mouse myeloma cell line that has lost immunoglobulin expression but permits the construction of antibodysecreting hybrid cell lines. J. Immunol. 123, 1548–1550 (1979). 43. Shi, S.R., Cote, R.J. & Taylor, C.R. Antigen retrieval immunohistochemistry: past, present, and future. J. Histochem. Cytochem. 45, 327–343 (1997). 44. Oliva, C. et al. The integrin Mac-1 (CR3) mediates internalization and directs Bacillus anthracis spores into professional phagocytes. Proc. Natl. Acad. Sci. USA 105, 1261–1266 (2008). 327