* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Whole body protein synthesis is an average of the synthesis rates
Homology modeling wikipedia , lookup
Protein design wikipedia , lookup
Intrinsically disordered proteins wikipedia , lookup
Protein folding wikipedia , lookup
Protein domain wikipedia , lookup
Protein structure prediction wikipedia , lookup
Bimolecular fluorescence complementation wikipedia , lookup
Protein mass spectrometry wikipedia , lookup
Western blot wikipedia , lookup
Protein purification wikipedia , lookup
Nuclear magnetic resonance spectroscopy of proteins wikipedia , lookup
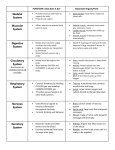
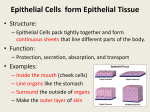
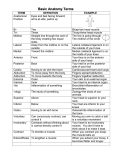
Sarcoplasmic Hypertrophy and Rep Range By Dan Moore One of the issues I still see when reading training articles is how the rep range dictates whether any hypertrophic response predominantly influences changes in size of the contractile components of muscle cells or the sarcoplasmic volume. The intent of this article is not to dispute that sarcoplasmic hypertrophy exists but to clarify if the rep range used will dictate which protein fraction will increase and whether or not the rep range specifically induces different changes to the nuclear domain of muscle fibers. There may very well be other factors such as metabolite accumulation and osmolality shifts within the cell that would give temporary changes to the muscle cell volume and size but since these are mostly temporary I did not feel they should be included in this article. What is the Sarcoplasm? Within the sarcoplasm there are soluble (or aqueous) components (making up 80 percent of it); composed of ions and soluble macromolecules like enzymes, carbohydrates, different salts and proteins, as well as a great proportion of RNA. This watery component can be more or less gel-like or liquid depending on the condition and the activity phases of the cell. In general, margin regions of the cell are gel-like and the cell's interior is liquid. The insoluble constituents of the sarcoplasm are organelles (such as the mitochondria, the chloroplast, lysosomes, peroxysomes, ribosomes), several vacuoles, cytoskeletons as well as complex membrane structures (e.g. sarcoplasmic reticulum). The muscle protein fraction that makes up the cytoplasm (sarcoplasm in a muscle cell) is made up of mostly enzymes participating in cell metabolism, such as the anaerobic energy conversion from glycogen to ATP, intracellular transport, and several other enzyme functions. This fraction adds up to about 25 or 30% of the total muscle protein versus the larger and more talked about structural protein (myofibril protein) makes up about 40%. Skeletal muscle protein When speaking of protein synthesis one of the things that first must be identified is which fraction of protein we are talking about. Whole body protein synthesis is an average of the synthesis rates of various proteins in different tissues of the body, skin, muscle, organs, and plasma. Mixed muscle protein is an average of the synthesis rates of total muscle proteins and includes mitochondrial, sarcoplasmic, structural components and connective tissue proteins. Myofibrillar protein is comprised of individual proteins such as myosin, actin, titin, tropomyosin, troponin, protein C, and some components of mitochondrial proteins. Sarcoplasmic proteins are mostly enzymes participating in cell metabolism. However, if the organelles within the muscle cells are broken, this protein fraction may also contain the metabolic enzymes localized inside the sarcoplasmic reticulum, mitochondria and lysosomes. Fractional Protein Synthesis It has now been known for some time that chronic elevation in protein synthesis above that of breakdown is of prime importance in skeletal muscle hypertrophy (1,2). It is this basis of understanding that we start this article off by looking into how feeding and exercise impacts fractional protein turnover. Years ago David Millward and Peter Bates identified that protein synthesis had a direct relationship to both feeding and fasting (3). They also noted that during tissue growth (from feeding) the maintenance of a constant composition necessitates the same absolute increase in synthesis for all proteins, both contractile (myofibril) and soluble (sarcoplasmic). This would mean that the increase in the synthesis rate for each protein will be an increasing proportion of the overall rate for the slowerturning-over proteins or in more simpler terms, the increase of both fractions are held within a ratio. Resistance training has shown very strong shifts in protein turnover (4,5) and most of the studies have used mixed muscle protein turnover as the gauge of effectiveness. Unfortunately this method does not show how resistance or dynamic exercise training differently affects each fraction. In a study looking at age affects of Protein Synthesis (6) it was noted that by the end of 2 weeks of weight-lifting exercise, MHC and mixed protein synthesis rates increased in both younger and older participants. The actin protein synthesis rate was increased after exercise in only the younger group. The magnitude of the exercise-induced increase in MHC and mixed protein synthesis rates was similar in the younger and older groups. In the younger group, the MHC and Actin (contractile) protein synthesis rate increased 83% and 78% respectively while the mixed muscle protein synthesis rate increased (102%). This study points to the identification that, as with feeding, all proteins are up regulated with resistance exercise. Now the interesting point was that the exercise protocol used seven weight-lifting exercises (Nautilus equipment) that included the chest press, inclined chest press, latissimus pull-down (wide and narrow grip), leg press, knee extension, knee flexion, and two free weight-lifting exercises that included seated overhead press and overhead triceps extension. Each participant completed ten weight-lifting exercise sessions: 2– 3 sets/day of the nine exercises listed above, 8–12 repetitions/set, 60–90% of maximum voluntary muscle strength. This was a pretty broad range of intensity and easily points out that the rep range itself isn’t the determining factor. To further illustrate that the intensity or rep range utilized does not change this ratio all too dramatically we can look at more recent work looking into the synthetic rates of fractional proteins with dynamic or resistance type exercise. Atherton et al. (7) used electrical stimulation with high frequency (HFS; 6x10 repetitions of 3 s-bursts at 100 Hz to mimic resistance training) to identify signalling present during increased protein synthesis. What he noted, significant to this article and discussion, was that HFS significantly increased myofibrillar and sarcoplasmic protein synthesis 3 h after stimulation 5.3 and 2.7 fold, respectively. Interestingly Bowtell (8) found that when the same total amount of ATP is turned over, exercise at 60, 75 and 90% of the one-repetition-maximum force results in exactly the same stimulation of muscle protein synthesis, suggesting that once all muscle fibers are recruited increases in tension above 65% cause no further stimulation in muscle protein synthesis. Even though I am not aware if the specific fractions were measured in the Bowtell study it would stand to reason that in light of the previous both fractions would be up regulated. In another study, Louis (9) subjects carried out 20 series of 10 repetitions (with a rest of 80 s after each series) on an isokinetic dynometer to evaluate if Creatine has an impact on anabolic signalling and protein synthesis. Again, in the realm of this article, what was found interesting was that this exercise increased the synthetic rates of myofibrillar and sarcoplasmic proteins by 2- 3 fold. Looking at dynamic exercise (one legged kicking), Miller (10) saw that the rates of protein synthesis in the exercised leg increased substantially by 6 h and peaked within 24 h in both myofibrillar and sarcoplasmic fractions, i.e. increases of 2.8 and 2-fold, respectively. The rates of myofibrillar and sarcoplasmic protein synthesis in the exercised muscle had fallen slightly by 48 h but were still significantly above the rates in the rested leg. By 72 h, the rates of both fractions had decreased. Our last look at fractional elevations will look at whether or not there is a fiber type dependency. In many animals the rate of protein synthesis is higher in slow-twitch, oxidative than fast-twitch, glycolytic muscles. To find if this held true for muscles in the human body a recent study (11) recruited nine healthy, young men and with a constant infusion looked at synthetic rates in the soleus, vastus lateralis and tricep. Type-1 fibers contributed 83 +/- 4% (mean +/-s.e.m.) of total fibers in soleus, 59 +/- 3% in vastus lateralis and 22 +/- 2% in triceps. The basal myofibrillar and sarcoplasmic protein fractional synthetic rates (FSR, % h(-1)) were 0.034 +/- 0.001 and 0.064 +/- 0.001 (soleus), 0.031 +/- 0.001 and 0.060 +/- 0.001 (vastus), and 0.027 +/- 0.001 and 0.055 +/- 0.001 (triceps). During amino acid infusion, myofibrillar protein FSR increased to 3-fold, and sarcoplasmic to 2-fold above basal values (P < 0.001), again showing that even within differing types of muscle tissue the ratio remains. What can be seen when reviewing these and many other papers on the subject is the response to resistance training of fractional elevation remains in line with the results of feeding, both are elevated but the slower turnover proteins (myofibrillar) generally show a larger magnitude in increase. Since these studies show that this holds true with resistance training, dynamic exercise and HFES, all utilizing differing intensities and work output, it seems unlikely that the rep range is the sole cause of any increase in sarcoplasmic fraction up-regulation. Nuclear Domains It is well established that satellite cell contribution is a very important factor in muscle hypertrophy (12,13). As a cell grows and increases it’s cytoplasmic volume the nucleus must maintain mRNA production for the entire area of increased size of cytoplasm (14). Since muscle cells are multi-nucleic, each nucleus controls mRNA over a finite area or its domain (14,15), hence the term nuclear domain. Skeletal muscle hypertrophy has shown to induce increases in myonuclei and or increases in domain size (16). Resistance training has reported a very broad range of increases in fiber hypertrophy (17). In several hypertrophy studies it appears that there is a limit that needs to be reached before domain size increases necessitates increased nuclei donation. Studies that have shown significant increase in hypertrophy, above 26%, also showed large additions of myonuclei in animals (1820) and in humans (21). Work in humans is rather limited but what can be seen in this smaller body of work is very interesting. Acutely, resistance training and resistance type training do not exert the same magnitude of response in humans as what is seen in smaller animals. A recent study (22) examining the acute effect of training showed less significant changes in donated nuclei and in fact after an acute bout of (a) fifty one legged ‘drop down’ jumps were performed from a stable platform of 45 cm, (b) eight sets of ten maximal eccentric knee extensions at –30 degree/s using an isokinetic dynamometer and (c) eight sets of ten maximal eccentric knee extensions at –180 degree/s using an isokinetic dynamometer, satellite cell proliferation did occur but there was no increased satellite cell donation. Apparently a single bout in humans is not enough to induce the same changes seen in smaller animals. Kadi looked at chronic application in a very interesting and telling look at training and detraining. (23). In this study he subjected 15 subjects to 3 months of progressive resistance training using a 6-12 RM. The various exercises were conducted in 4–5 sets, in the first weeks (training sessions 0–5) exercises involved 10–12 RM loads, followed by 10 RM loads in early weeks (training sessions 6–15), heavier loads of 6–10 RM in the later weeks (training sessions 16–30), and very heavy loads of 6–8 RM in the final weeks (training sessions 31–38). The subjects then detrained for 3 months. Satellite cell activity increased significantly over the entire training period. Reaching significance at around 30 and again at the 90-day marks. Hypertrophy of fiber increased at 30 days and 90 days, 6.7% and 17% respectively. Interestingly though was the observation that the area controlled by each nuclei was virtually unchanged over the entire training period. This clearly illustrates that the rep range was not the primary inducer of hypertrophy or domain volume changes since the fiber size and satellite cell count increased no matter the rep range. It also clearly indicates that the duration of chronic training is a key element in both hypertrophy and satellite cell activity. An earlier study by the same researcher (24) saw a concomitant increase in satellite cell count and an increase in myonuclei donation over a 10-week training protocol in female athletes. The muscle examined in this study was the trapezius so it could be that the increase could be muscle specific, as it’s been shown that the trapezius has a higher androgen receptor content (25). This may have also been another reason why the previously cited work by Kadi showed such a difference as the biopsies were taken from the vastus lateralis. In summary it is very common to see studies reflecting both increased protein synthesis and hypertrophy with a myriad of rep ranges and resistance training protocols. The extent of hypertrophy may be a direct reflection in increased translational efficiency or an increase in pre-translational abundance of mRNA. The differences may be owing to the training status of the individual and not necessarily the rep range used in the resistance training routine. Although it appears that the rep range will have an impact on metabolic shifts in isoform content this does not change the sarcoplasmic vs. contractile protein synthesis ratio but merely dictates which fiber type will experience the greater amount of hypertrophy. As stated in Rennie’s 2004 review (26), it may take 20 weeks of resistance training to increase hypertrophy by 20%. This coincides very well with the research presented in this article, as it appears that the change in fiber size has a direct correlation to when satellite cells donate their nuclei for continued domain regulation. Therefore moderate increases in nuclear domain are very possible without the aided donation of nuclei from satellite cells and this does not appear to be rep range dependant. A statement can also be made that the results seen in small animals and humans may be very different. This may be owing to the extent of damage that is seen and how hypertrophy and necrotic damage stimulate the satellite pool differently but that is beyond the scope of this article. Dan Moore Hypertrophy-Research.com (1)Changes in rates of protein synthesis breakdown during hypertrophy of the anterior and posterior latissimus dorsi muscles. Biochem. J. 176:407–17 (2)Protein turnover during skeletal muscle hypertrophy.Fed. Proc. 39:42–47 (3)Synthesis rates of myofibrillar and sarcoplasmic protein fractions in different muscles and the changes observed during postnatal development and in response to feeding and starvation Biochem. J. (1983) 214,587-592 (4)Increased rates of muscle protein turnover and amino acid transport after resistance exercise in humans. Am J Physiol Endocrinol Metab 268: E514–E520, 1995. (5)Changes in human muscle protein synthesis following resistance exercise. J Appl Physiol 73: 1383– 1388,1992. (6)Resistance exercise acutely increases MHC and mixed muscle protein synthesis rates in 78–84 and 23– 32 yr olds Am J Physiol Endocrinol Metab 278: E620–E626, 2000. (7) Selective activation of AMPK-PGC-1alpha or PKB-TSC2-mTOR signaling can explain specific adaptive responses to endurance or resistance training-like electrical muscle stimulation. FASEB J. 2005 May;19(7):786-8. Epub 2005 Feb 16. (8) Stimulation of human quadriceps protein synthesis after strenuous exercise: no effects of varying intensity between 60 and 90% of one repetition maximum (1RM). J Physiol 547.P, P16. (9) No effect of creatine supplementation on human myofibrillar and sarcoplasmic protein synthesis after resistance exercise. Am J Physiol Endocrinol Metab. 2003 Nov;285(5):E1089-94. (10)Coordinated collagen and muscle protein synthesis in human patella tendon and quadriceps muscle after exercise.J Physiol. 2005 Sep 15;567(Pt 3):1021-33. (11)Protein synthesis rates in human muscles: neither anatomical location nor fibre-type composition are major determinants. J Physiol. 2005 Feb 15;563(Pt 1):203-11. Epub 2004 Dec 20. (12)Gamma irradiation prevents compensatory hypertrophy of overloaded mouse extensor digitorum longus muscle. J. Appl. Physiol. 73:2538–43 (13)Cellular molecular responses to increased skeletal muscle loading after irradiation. Am. J. Physiol. Cell Physiol. 283:C1182–95 (14)The control of cell mass and replication. The DNA unit-a personal 20 year study. Early Hum Dev 12, 211-239. (15) Nuclear Domains in Muscle Cells. Cell 59, 771-772 (16)Regulation of Skeletal muscle fiber size, shape and function. J Biochem (Suppl. 1), 123-133 (17) Exercise, protein metabolism, and muscle growth. Int J Sport Nutr Exerc Metab. 2001 Mar;11(1):109-32. Review. (18)Morphometric analyses on the muscles of exercise trained and untrained dogs. Am J Anat. 1983 Mar;166(3):359-68. (19)Plasticity of myonuclear number in hypertrophied and atrophied mammalian skeletal muscle fibers. J Appl Physiol. 1995 May;78(5):1969-76. (20)Modulation of myonuclear number in functionally overloaded and exercised rat plantaris fibers.J Appl Physiol. 1999 Aug;87(2):634-42. (21)Is Hypertrophy limited in elderly muscle fibers? A comparison in elderly and young strength-trained men. Basic Appl Myol 1998; 8, 419-427 (22)Changes in satellite cells in human skeletal muscle after a single bout of high intensity exercise. J Physiol. 2004 Jul 1;558(Pt 1):333-40. (23)The effects of heavy resistance training and detraining on satellite cells in human skeletal muscles. J Physiol. 2004 Aug 1;558(Pt 3):1005-12. (24)Concomitant increases in myonuclear and satellite cell content in female trapezius muscle following strength training. Histochem Cell Biol 2000; 113, 99-103. (25)The expression of androgen receptors in human neck and limb muscles: effects of training and selfadministration of androgenic-anabolic steroids. Histochem Cell Biol. 2000 Jan;113(1):25-9. (26)Control of the Size of the Human Muscle Mass Annu. Rev. Physiol. 2004. 66:799–828