* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Anatomy and Physiology of the Neuromuscular Junction
Survey
Document related concepts
Organ-on-a-chip wikipedia , lookup
NMDA receptor wikipedia , lookup
SNARE (protein) wikipedia , lookup
Cyclic nucleotide–gated ion channel wikipedia , lookup
Cytokinesis wikipedia , lookup
List of types of proteins wikipedia , lookup
Endomembrane system wikipedia , lookup
Cell membrane wikipedia , lookup
Node of Ranvier wikipedia , lookup
Mechanosensitive channels wikipedia , lookup
Signal transduction wikipedia , lookup
Action potential wikipedia , lookup
Transcript
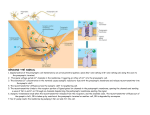
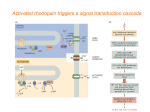
Anatomy and Physiology of the Neuromuscular Junction Anatomy We stimulate skeletal muscle contraction voluntarily. Electrical signals from the brain through the spinal cord travel through the axon of the motor neuron. The axon then branches through the muscle and connects to the individual muscle fibers at the neuromuscular junction. The folded sarcolemma of the muscle fiber that interacts with the neuron is called the motor end-plate; the folded sarcolemma increases surface area contact with receptors. The ends of the branches of the axon are called the synaptic terminals, and do not actually contact the motor end-plate. A synaptic cleft separates the synaptic terminal from the motor end-plate, but only by a few nanometers. Communication occurs between a neuron and a muscle fiber through neurotransmitters. Neural excitation causes the release of neurotransmitters from the synaptic terminal into the synaptic cleft, where they can then bind to the appropriate receptors on the motor endplate. The motor end-plate has folds in the sarcolemma, called junctional folds, that create a large surface area for the neurotransmitter to bind to receptors. Generally, there are many folds and invaginations that increase surface area including junctional folds at the motor endplate and the T-tubules throughout the cells. Physiology The neurotransmitter acetylcholine is released when an action potential travels down the axon of the motor neuron, resulting in altered permeability of the synaptic terminal and an influx of calcium into the neuron. The calcium influx triggers synaptic vesicles, which package neurotransmitters, to bind to the presynaptic membrane and to release acetylcholine into the synaptic cleft by exocytosis. Review the section of this course about membranes if you need a refresher. The balance of ions inside and outside a resting membrane creates an electric potential difference across the membrane. This means that the inside of the sarcolemma has an overall negative charge relative to the outside of the membrane, which has an overall positive charge, causing the membrane to be polarized. Once released from the synaptic terminal, acetylcholine diffuses across the synaptic cleft to the motor end-plate, where it binds to acetylcholine receptors, primarily the nicotinic acetylcholine receptors. This binding causes activation of ion channels in the motor end-plate, which increases permeability of ions via activation of ion channels: sodium ions flow into the muscle and potassium ions flow out. Both sodium and potassium ions contribute to the voltage difference while ion channels control their movement into and out of the cell. As a neurotransmitter binds, these ion channels open, and Na+ ions enter the membrane. This reduces the voltage difference between the inside and outside of the cell, which is called depolarization. As acetylcholine binds at the motor-end plate, this depolarization is called an end-plate potential. It then spreads along the sarcolemma, creating an action potential as voltage-dependent (voltage- gated) sodium channels adjacent to the initial depolarization site open. The action potential moves across the entire cell membrane, creating a wave of depolarization. After depolarization, the membrane needs to be returned to its resting state. This is called repolarization, during which sodium channels close and potassium channels open. Because positive potassium ions (K+) move from the intracellular space to the extracellular space, this allows the inside of the cell to again become negatively charged relative to the outside. During repolarization, and for some time after, the cell enters arefractory period, during which the membrane cannot become depolarized again. This is because in order to have another action potential, sodium channels need to return to their resting state, which requires an intermediate step with a delay. Propagation of an action potential and depolarization of the sarcolemma comprise the excitation portion ofexcitation-contraction coupling, the connection of electrical activity and mechanical contraction. The structures responsible for coupling this excitation to contraction are the T tubules and sarcoplasmic reticulum (SR). The T tubules are extensions of the sarcolemma and thus carry the action potential along their surface, conducting the wave of depolarization into the interior of the cell. T tubules form triads with the ends of two SR called terminal cisternae. SRs, and especially terminal cisternae, contain high concentrations of Ca2+ ions inside. As an action potential travels along the T tubule, the nearby terminal cisternae open their voltagedependent calcium release channels, allowing Ca2+ to diffuse into the sarcoplasm. The influx of Ca2+ increases the amount of calcium available to bind to troponin. Troponin bound to Ca2+ undergoes a conformational change that results in tropomyosin moving on the actin filament. When tropomyosin moves, the myosin binding site on the actin is uncovered. This continues as long as excess Ca2+ is available in the sarcoplasm. When there is no more free Ca2+ available to bind to troponin, the contraction will stop. To restore Ca2+ levels back to a resting state, the excess Ca2+ is actively transported back into the SR. In a resting state, Ca2+ is retained inside the SR, keeping sarcoplasmic Ca2+ levels low. Low sarcoplasmic calcium levels prevent unwanted muscle contraction.