* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Elevated Level of Nuclear Protein Kinase C in
Cell nucleus wikipedia , lookup
Endomembrane system wikipedia , lookup
Signal transduction wikipedia , lookup
Cellular differentiation wikipedia , lookup
Cell culture wikipedia , lookup
Tissue engineering wikipedia , lookup
Organ-on-a-chip wikipedia , lookup
Cell encapsulation wikipedia , lookup
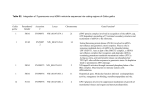
[CANCER RESEARCH 52. 3750-3759. July I. 1992) Elevated Level of Nuclear Protein Kinase C in Multidrug-resistant MCF-7 Human Breast Carcinoma Cells Sung Ae Lee, James W. Karaszkiewicz, and Wayne B. Anderson1 Laboratory of Cellular Oncology, National Cancer Institute, NIH, Bethesda, Maryland 20892 ABSTRACT Previous studies have demonstrated elevated levels of protein kinase C (PKC) activity in multidrug-resistant human breast carcinoma MCF7/ADR cells compared to control drug-sensitive MCF-7/WT cells (R. L. Fine, J. Patel, and B. A. Chabner, Proc. Nati. Acad. Sci. USA, 85:582586, 1988). In our present studies, immunohistochemical localization analysis using a polyclonal PKC antibody recognizing the a, ¡i.and y subtypes of PKC demonstrates that immunoreactivity is enhanced in MCF-7/ADR cells, with pronounced staining noted in the nuclear region. Other studies with purified nuclei isolated from MCF-7/ADR cells also show a marked increase in the intensity of immunostaining for PKC when compared to nuclei prepared from control MCF-7/WT cells. Western blot analysis of proteins extracted from purified nuclear preparations further establishes an increase in PKC enzyme protein associated with the nuclear fraction of MCF-7/ADR cells. Subcellular fractionation stud ies also indicate that MCF-7/ADR cells have 4-8 times higher nuclear PKC activity compared to that of control MCF-7/WT cells. MCF-7/ ADR cells also possess 3-5-fold elevated cytosolic PKC activity, while a <2-fold increase is found in PKC activity associated with the plasma membrane fraction of MCF-7/ADR cells. Examination of these extracts with PKC isotype-specific antisera, as well as by DEAE-cellulose chromatography, reveals that nuclei prepared from MCF-7/ADR cells contain markedly elevated amounts of a slightly altered form of PKCa. These results suggest that elevated levels of a modified form of PKCa at the nucleus may play a role in modulating nuclear events to promote the development of multidrug resistance in MCF-7 cells. INTRODUCTION nisms are (a) alterations in glutathione metabolism (8-11), (b) a decrease in metabolic activation of drug ( 12), and (c) differ ential oxygen free-radical susceptibility (13). It also has been reported that PKC activity is increased in several cell types exhibiting the MDR phenotype, including Adriamycin-resistant MCF-7 human breast carcinoma cells (14). The enhanced levels of PKC in MCF-7 cells resistant to Adriamycin and the mod ulation of the drug-resistant phenotype by agents which alter PKC activity (i.e., TPA, which first activates and then subse quently down-regulates PKC, as well as H-7 [l-(5-isoquinolinesulfonyl)-2-methylpiperazine] PKC inhibitor) suggest a role for PKC in multidrug resistance (14-17). Thus, it is of impor tance to characterize the biological and regulatory properties of PKC found elevated in multiple drug-resistant cell types. To better define a possible role of PKC in the modulation of the multidrug resistance phenotype, we have assessed changes in PKC activity, subcellular distribution, and isotype pattern in drug-sensitive MCF-7/WT cells and in drug-resistant MCF-7/ ADR cells. The MCF-7/ADR cells were found to possess markedly elevated levels of a modified form of PKCa in the nucleus. These results suggest that an increase in a specific form of PKCa in the nucleus of MCF-7 human breast carci noma cells may play a role in modulating nuclear events such as altered transcription to promote the development of multidrug resistance in MCF-7 cells. MATERIALS Development of resistance to cancer chemotherapeutic agents such as doxorubicin (Adriamycin) and vinblastine severely lim its the use of these drugs in cancer treatment. A particular phenotype of resistance, called multidrug-resistance (MDR),2 exhibits a broad spectrum of resistance to anticancer drugs derived from natural products or their derivatives including anthracyclines, Vinca alkaloids, epipodophyllotoxins, and actinomycin D (l, 2). Although the mechanism associated with the development of multidrug resistance is still unclear, a common feature of multidrug resistance is a net decrease in the intracellular accumulation of drugs as a consequence of en hanced drug efflux and the overexpression of a membraneassociated P-glycoprotein (3-7). However, several lines of evi dence suggest that mechanisms in addition to a net decrease in drug accumulation may be also involved in modulating multidrug resistance. Among other possible contributing mecha- AND METHODS Materials. Doxorubicin (Adriamycin) and histone HI (type HIS. lysine-rich histone) were purchased from Sigma (St. Louis, MO). Leupeptin, »pmtimn. (4-amidinophenyl)methanesulfonyl fluoride (watersoluble form of PMSF), soybean trypsin inhibitor, pepstatin A, dithiothreitol, biotinylated goat anti-rabbit IgG, alkaline phosphatase-conjugated streptavidin, nitroblue tetrazolium chloride, and S-bromo-4chloro-3-indolyl phosphate were obtained from Boehringer-Mannheim (Indianapolis, IN). DNase II and CHAPS detergent were from Calbiochem (La Jolla, CA). 1,2-Dioleoyl-sn-glycerol and phosphatidylserine were from Avanti Polar Lipids (Pelham, AL). [-Y-"P]ATP was pur chased from New England Nuclear (Boston, MA). Cell Lines. MCF-7 human breast cancer cell lines (drug-sensitive MCF-7/WT cells and multidrug-resistant MCF-7/ADR cells) were generously provided by Dr. Kenneth Cowan (National Cancer Institute, NIH, Bethesda, MD). Cells were grown in Richter's IMEM supple mented with 2 HIM L-glutamine, 2 mg/ml L-proline, 50 Mg/m' gentamicin, and 10% fetal bovine serum (Flow Laboratories, New York, NY) at 37°Cunder a humidified atmosphere of 5% COj in air. Cells were passaged twice weekly and monitored routinely for their resistance to Adriamycin. To maintain a highly drug-resistant cell population the multidrug-resistant MCF-7/ADR cells were periodically reselected by growing them in the presence of increasing concentrations of Adria mycin (7-10 /JM) for approximately 2-month periods of time. Experi ments were carried out using cells between passages 5 and 20. Another set of independently derived drug-sensitive and drug-resistant MCF-7 cell lines was kindly provided by Dr. Grace Yeh [National Cancer Institute; designated as MCF-7(GY) cells to distinguish from the MCF7 cell lines obtained from Dr. Kenneth Cowan). Drug-sensitive MCF7(GY)/WT cells and multidrug-resistant MCF-7(GY)/W0.3 and MCF7(GY)/WIO cells were grown in RPMI 1640 (GIBCO, Gaithersburg, 3750 Received 3/3/92; accepted 4/23/92. The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked advertisement in accordance with 18 U.S.C. Section 1734 solely to indicate this fact. 1To whom requests for reprints should be addressed, at Laboratory of Cellular Oncology, National Cancer Institute, NIH, Bldg. 36. Room 1D22, Bethesda, MD 20892. 2The abbreviations used are: MDR, multidrug resistance; PKC. protein kinase C; TPA. ^-O-tetradecanoylphorbol-U-acetate; CHAPS, 3-[(3-cholamidopropyl)dimethylammonio)propanesulfonate; MTT. 3-(4.5-dimethyl(thiazol-2-yl)3.5-diphery|tetradium bromide; EGTA, ethyleneglycol-bisW-aminoethyl ether)A/,A/,.V'..\"-tetraaceticacid; D-PBS. Dulbecco's phosphate buffered saline; IMEM, improved minimum essential medium; PBS, phosphate-buffered saline; BSA, bovine serum albumin; SDS. sodium dodecyl sulfate; IC50, drug concentration required for SO1";cytotoxicity: CM-free. Ca2*- and Mg2*-free. Downloaded from cancerres.aacrjournals.org on June 17, 2017. © 1992 American Association for Cancer Research. ELEVATED NUCLEAR PKC AND DRUG RESISTANCE MD) supplemented with 2 HIML-glutamine and 10% fetal bovine serum (Flow Laboratories) at 37°Cunder a humidified atmosphere of 5% CO2 in air. PKC Antibodies. Four different polyclonal PKC antibodies were used in these studies. Antibodies were raised in rabbits against keyhole limpet hemocyanin-conjugated synthetic peptides corresponding (a) to the NH2-terminal pseudosubstrate region common to isotypes a, ß, and y (FARKGALRQKNV) and thus pan-specific for these isotypes, (b) to the a isotype-specific hinge region peptide (KLGPAGNKVISPSED), (c) to the ß isotype-specific COOH-terminal region (SEFEGFSFVNSEFLKPEVKS), and (d) to the 7 isotype-specific COOH-terminal re gion (AEFQGFTYVNPDFVH) of PKC. The isotype specificity was screened using extracts prepared from COS-7 cells transfected with the «,ß,and 7 isotypes of PKC. Each antiserum was found to be specific for the PKC isotype indicated and exhibited no cross-reactivity with the other PKC isotypes tested. Immunohistochemical Localization of PKC. MCF-7/WT cells and MCF-7/ADR cells grown on Lab-Tek Permanox tissue culture chamber slides were washed with PBS, fixed with 4% formaldehyde/0.05% glutaraldehyde in PBS, and then permeabilized with 0.1% Triton X100 in PBS. The permeabilized cells were sequentially incubated with 10% normal horse serum, 10% normal goat serum, and 2% BSA (globulin-free) in PBS for 30 min each to block nonspecific IgG binding. Then, the cells were incubated overnight at 4°Cwith PKC antiserum (pan-specific for a, ß,and 7 isotypes of PKC) in PBS containing 2% normal goat serum, 1% BSA (globulin-free), and 0.05% Triton X-100. The cells then were rinsed with PBS and subsequently incubated with biotinylated goat anti-rabbit IgG in PBS containing 1% BSA (globulinfree) and 0.01 % Tween 20. The immunoreactivity to PKC was localized by incubation with alkaline phosphatase-conjugated avidin D and sub sequent color development by incubation with Vector ABC kit (Vector Laboratories, Burlingame, CA). Preparation of Nuclear-Cytoskeletal Components. Intact MCF-7/WT cells and MCF-7/ADR cells grown on Lab-Tek Permanox tissue culture chamber slides were rapidly washed with warm (30°C)PBS and ex tracted for 10-14 min at 30°Cby incubation with buffer solution containing 0.1 M piperazine-A',Ar'-bis(2-ethanesulfonic acid) (pH 6.9), sample was 0.4 mM for each. Phosphatidylserine (25 /jg) and diolein (2.5 Mg)were added to some tubes in a volume of 20 n\ to demonstrate phospholipid-dependent protein kinase activity. After incubation for 6-10 min at 30°C,50 n\ of aliquots of the reaction mixture were spotted onto paper strips (Whatman P-81 ion exchange chromatography pa per), and the strips were immediately immersed into 75 mM phosphoric acid. The P-81 papers were washed at least 4 times with 75 mM phosphoric acid and air dried, and radioactivity retained on the papers was determined. Protein kinase C activity was calculated from the difference in 32Pincorporated into histone in the presence and absence of added phospholipids and calcium and was expressed as '2P incorporated/min/mg protein. Colorimetrie MTT Test for Cytotoxicity to Adriamycin. MCF-7 cells were seeded in 24-well plates (Costar, Cambridge, MA) at a density of approximately 25,000 cells/well in 1MEM as described above. After 24 h the medium was removed and I nil aliquots of IMl1'M containing various concentrations of doxorubicin and supplemented with 2 mM Lglutamine, 2 mg/ml i.-proline, 50 pg/ml gentamicin and 10% heatinactivated fetal bovine serum were added to each well. After 48 h of incubation at 37°C,100 nl of MTT at a concentration of 5 mg/ml in D-PBS were added to each well, and the cultures were further incubated for 2 h at 37°C.The reaction was terminated by removal of the medium along with the MTT dye, and the formazan precipitate formed was dissolved in 1 ml of dimethyl sulfoxide. The relative number of viable cells remaining after Adriamycin treatment was estimated by the change in absorbance at 590 nm. The relative percentage of surviving cells was calculated asAbsorbance in wells treated with doxorubicin + Absorb ance in control wells without doxorubicin x 100 Western Blot Analysis of PKC. Subconfluent MCF-7 cells grown in IMEM were rapidly washed twice with ice-cold Ca:*- and Mg;+-free (CM-free) D-PBS. A hot SDS total cell extract then was prepared by immediately scraping the cells into boiling SDS-polyacrylamide gel electrophoresis sample buffer and drawing through a 23-gauge needle 10 times to shear DNA (19). The extracts were then boiled for 5 min. Electrophoresis of the extracted proteins was carried out on 8% SDSpolyacrylamide gel electrophoresis minigels unless otherwise described. The protein bands then were transferred electrophoretically to Immobilon-P membrane (Millipore, Bedford, MA). Following electrotransfer, the membrane was incubated with 2% (w/v) Carnation nonfat dry milk in CM-free D-PBS for 30 min at room temperature to block nonspecific IgG binding sites and then washed with CM-free D-PBS for 10 min. The membrane then was incubated overnight at 4°Cwith protein kinase C antiserum diluted 1:500 in CM-free D-PBS containing 2% nonfat dry milk. The membrane exposed to antiserum then was washed sequentially with 0.25% Tween 20 in CM-free D-PBS for 10 min, with 0.05% Tween 20 in CM-free D-PBS twice (10 min each wash), and finally with CM-free D-PBS for 10 min at room tempera ture. After primary antiserum incubation and washings, the membrane was incubated with biotinylated secondary antibody diluted 1:1000 in CM-free D-PBS containing 2% nonfat dry milk for 1.5-2.5 h at room temperature and then washed as described above. The washed mem brane then was incubated with alkaline phosphatase-conjugated streptavidin diluted 1:1000 in CM-free D-PBS containing 2% nonfat dry milk for 30 min at room temperature and again washed as described above. PKC was visualized by incubation with 0.4 mM nitroblue tetrazolium-0.4mM 5-bromo-4-chloro-3-indolyl-phosphatein 100 mM TrisCl (pH 9.5) buffer solution containing 100 mM NaCl and 10 mM MgCl2 at room temperature for 10-20 min. To terminate the color develop ment reaction the membrane was washed with 50 mM EDTA, subse quently washed with deionized 11 (). and then air dried. Protein Determination and Data Expression. Protein concentration was determined by Bradford protein assay (20) using BSA as standard. Data are expressed as mean ±SEM. Error bars smaller than the symbols or lines used in the figures have been omitted. 5 mM MgCb, 0.2 mM EGTA, 4 M glycerol, 100 ng/m\ leupeptin, and 0.1% Triton X-100. The solubilized fraction was quickly removed to leave nuclear-cytoskeletal structures attached to the substratum. The remaining nuclei and cytoskeletal structures were washed twice with cold PBS and subsequently processed for the immunohistochemical localization of PKC as described above. Subcellular Fractionation. Cells were washed twice with ice-cold Ca2+and Mg:*-free D-PBS and then harvested by scraping from culture dishes into ice-cold Equilibration Buffer [20 mivi Tris-HCl (pH 7.5) containing 2 m\i EDTA, 2 mM EGTA, 2 mM dithiothreitol, and protease inhibitors (100 ;ig/ml leupeptin, 50 iig/ml aprotinin, 50 p%/ ml 4-amidinophenyl-methanesulfonyl fluoride, 10 iig/ml soybean trypsin inhibitor, and 100 ng/ml pepstatin A)], and then disrupted by 100 strokes (pestle B) with a Dounce homogenizer. Crude nuclei were isolated by centrifugation of total cell homogenate at 1000 x g for 5 min. This crude nuclear pellet was subsequently purified by sucrose density centrifugation as described previously (18). The purity of the nuclear preparations was determined by electron microscopy and was routinely monitored by phase contrast microscopy after staining with méthylène blue. The plasma membrane-enriched fraction was prepared by centrifugation of the 1,000 x g supernatant at 10,000 x g for 20 min. The cytosolic fraction was isolated by centrifugation of the 10,000 x g supernatant at 100,000 x g for 60 min. For PKC activity measure ment the total cell homogenate, purified nuclear fraction, and plasma membrane-rich fractions were solubilized with 1% (w/v) CHAPS on ice overnight. Assay of PKC Activity. The reaction was initiated by addition of 13 ng of protein sample to a reaction mixture containing 20 mM Tris-Cl (pH 7.5), 1 mM CaCl2, 10 mM MgCl2, 100 /¿M [7-"P]ATP (3 x 10" RESULTS cpm), 2 mM dithiothreitol, and 200 pg/m\ histone type III-S or 4 ¿IM PKC substrate peptide [(Ser2i)PKC( 19-31)] (GIBCO) as phosphoacImmunohistochemical Localization of PKC in MCF-7/WT and localization of ceptor substrate in a total volume of 100 n\. The final concentration of MCF-7/ADR Cells. The immunohistochemical PKC was determined with rabbit anti-peptide antiserum panEDTA and EGTA in the reaction mixture contributed by the protein 3751 Downloaded from cancerres.aacrjournals.org on June 17, 2017. © 1992 American Association for Cancer Research. ELEVATED NUCLEAR PKC AND DRUG RESISTANCE specific for the o, ß,and 7 isotypes of PKC prepared as described in "Materials and Methods." In intact MCF-7 cells, multidrug-resistant MCF-7/ADR cells showed a markedly en hanced immunoreactivity to PKC compared to that of drugsensitive MCF-7/WT cells (Fig. 1). The most pronounced difference was found in the nuclear region. A dense immunostaining was found in the nuclear region of MCF-7/ADR cells while little immunostaining was present in the perinuclear region of MCF-7/WT cells. This finding was confirmed in studies using isolated nuclei (Fig. 2). Nuclei isolated from MCF7/ADR cells showed a marked increase in intensity of immuno staining for PKC compared to nuclei prepared from drugsensitive MCF-7/WT cells. A similar result was observed with a isotype-specific PKC antibody, while no significant difference in the intensity of immunostaining for ß-isotypePKC was found in MCF-7/WT and MCF-7/ADR cells (data not shown). Subcellular Distribution of PKC Activity in MCF-7/WT Cells and MCF-7/ADR Cells. It has been previously demonstrated that PKC activity is elevated in MCF-7/ADR cells compared to MCF-7/WT cells (14). To better characterize the elevated PKC activity found in MCF-7/ADR cells, the subcellular dis tribution of PKC activity in MCF-7/WT and MCF-7/ADR cells was compared (Table 1). In the process of subcellular traci¡on;iiion. the nuclear pellets isolated from MCF-7/WT and MCF-7/ADR cells were further purified as described by Thomas et al. (18). The purity of the nuclear fraction was verified by electron microscopic examination (data not shown). The total cell homogenate of MCF-7/ADR cells exhibited about a 4-fold increase in total PKC activity compared to that of MCF-7/WT cells. The most striking difference in total PKC activity, and also in specific activity, was found in the nuclear fraction. The nuclear fraction isolated from MCF-7/ADR cells exhibited a 4-8-fold higher PKC specific activity when com pared to those of MCF-7/WT cells. Similar results were ob served when the nuclear preparation was isolated following disruption of the cells in homogenization buffer containing detergent such as 0.05% Triton X-100 or containing 0.5 M hexylene glycol (data not shown). A higher level (3-5-fold) of PKC activity was also found in the cytosolic fraction of MCF7/ADR cells, while a lesser increase (<2-fold) was noted in plasma membrane-associated PKC activity of MCF-7/ADR cells. To exclude the possibility that the significant pool of nuclear PKC activity found in the MCF-7/ADR cells might originate from residual contamination from the cytosolic pool, we carried out a time course analysis of changes in the distri bution and down-regulation of PKC in the different subcellular fractions in response to treatment of these cells with 1 ^M TPA (Fig. 3). Exposure of MCF-7/ADR cells to l /tM TPA resulted MRS Control PKC Aby MCF-7/ADR MCF-7/WT Fig. 1. Immunohistochemical localization of protein kinase C in intact drug-sensitive MCF-7/WT and multidrug-resistant MCF-7/ADR human breast carcinoma cells. MCF-7/WT and MCF-7/ADR cells were fixed and analyzed for PKC by immunohistochemical staining as described in "Materials and Methods." Primary antibody used was rabbit anti-peptide antiserum (pan-specific for MHz-terminal pseudosubstrate region of a. ti, and y isotype of PKC). In the control group, normal rabbit serum was used in place of PKC antibody. 3752 Downloaded from cancerres.aacrjournals.org on June 17, 2017. © 1992 American Association for Cancer Research. ELEVATED NUCLEAR PKC AND DRUG RESISTANCE MRS Control PKC Aby MCF-7/WT MCF-7/ADR Fig. 2. Immunohistochemical localization of protein kinase C in the nuclear-cytoskeletal fraction of drug-sensitive MCF-7/WT and multidrug-resistant MCF-7/ ADR human breast carcinoma cells. The nuclear-cytoskeletal fraction was prepared as described in "Materials and Methods." Rabbit anti-peptide antiserum (panspecific for NHj-terminal pseudosubstrate region of «,/i, and y isotypes of PKC) was used in this study, while normal rabbit serum was used as a control. The immunoreactivity to PKC visualized with a Vector ABC kit was photographed with an Olympus Vanox microscope at x 400. Table 1 Subcellular distribution of protein kinase C activity in control drug-sensitive MCF-7/ WT and multidrug-resistant MCF-71 ADR cells Subcellular fractionation and PKC activity measurement were carried out as described in "Materials and Methods." Data are expressed as mean ±SEM. Protein kinase C activity units (nmol/min/108 cells)MCF-7/ADR32.51protein)Fold Subcellular fractionTotal homogenate Nucleus Plasma membrane CytosolMCF-7/WT8.79 ±1.37 0.78 ±0.08 1.21 ±0.08 7.28 ±0.64Total ±2.03 5.75 ±0.35 2.66 ±0.37 34.84 ±4.23Specific in a rapid loss of PKC activity from the cytosolic fraction within 30 min (to ~20% of control). Concomitantly, there was a pronounced increase in plasma membrane-associated PKC ac tivity (to ~370% of control). In contrast, the nuclear pool of PKC activity was not appreciably altered at early (30- and 90min) time periods following exposure of the cells to TPA, although a rise (to ~160% of control) in nuclear PKC activity was noted at 3 h after the addition of TPA. Activity analysis also revealed that the time course and extent of down-regulation of PKC in the different subcellular fractions in response to prolonged exposure to TPA also are different. Cytosolic PKC was translocated and diminished within 30 min of exposure to TPA, although a residual (~20% of control) level of cytosolic activity (nmol/min/mg increase3.7 increase4.5 ±0.04 ±0.07 7.4 0.27 ±0.03 1.37 ±0.08 0.89 ±0.04 1.30 ±0.03 2.2 4.8MCF-7/WT0.250.46 ±0.04MCF-7/ADR1.12 2.18 ±0.26Fold 5.1 1.5 4.7 PKC activity remained throughout the 24-h period of TPA treatment. The dramatic increase in plasma membrane-associ ated PKC was transient, and within 90 min after TPA treatment the PKC activity in the plasma membrane fraction had fallen by 60%. The plasma membrane PKC activity continued to show a drop through the remainder of the 24-h treatment period, but with only a maximal decrease to 40% of control. The nuclear pool of PKC, however, was completely down-regulated within 12 h of TPA treatment, and no PKC activity could be detected in the nuclear fraction at either the 12- or 24-h time periods. The observed differences in the time course and extent of TPAinduced redistribution and down regulation of PKC associated with the cytosolic, plasma membrane, and nuclear subcellular 3753 Downloaded from cancerres.aacrjournals.org on June 17, 2017. © 1992 American Association for Cancer Research. ELEVATED NUCLEAR PKC AND DRUG RESISTANCE the subsequent detergent-solubilized nuclear PKC activity in both drug-sensitive MCF-7/WT cells and multidrug-resistant MCF-7/ADR cells. These results indicate that the majority of PKC remains with the insoluble nuclear fraction and can be removed by detergent. Correlation between Nuclear PKC Activity and Drug Resist ance. The profound increase in nuclear PKC activity observed in multidrug-resistant MCF-7/ADR cells led us to investigate whether the level of nuclear PKC activity might correlate with the degree of drug resistance in MCF-7 cells. For this study, we used three different MCF-7 cell lines with different levels of drug resistance: one control MCF-7/WT cell line; and two drug-resistant MCF-7/ADR cell lines designated as MCF-7/ ADRC| cells and MCF-7/ADRjm cells. The relative drug resist ance of the three MCF-7 cell lines was determined by comparing the chemosensitivity of these cells to the cytotoxic effect of doxorubicin (Adriamycin) as determined by the MTT colori metrie assay as described in "Materials and Methods." Under the given culture and assay conditions, MCF-7/ADRjm cells 12 18 24 TIME (hours) Fig. 3. Effect of TP A treatment of MCF-7/ADR cells on the distribution and down-regulation of protein kinase C activity found in the cytosolic, plasma membrane, and nuclear subcellular fractions. MCF-7/ADR cells were treated with l /JM TPA for the indicated periods of time, the cells were harvested by scraping, and cytosolic, plasma membrane, and nuclear fractions were prepared as described in "Materials and Methods." Assays for PKC activity present in the subcellular fractions were carried out as described in "Materials and Methods" using PKC substrate peptide [(Ser")PKC( 19-31)] (GIBCO) as phosphoacceptor substrate. Purified nuclei and cytosol were prepared as described in "Materials and Methods." Table 2 Detergent-solubilized PKC activity remaining in nuclei isolated from MCF-7/WTand MCF-7/ADR cells following either high salt concentration washing or DNase II digestion of the nuclear preparations Purified nuclei isolated from MCF-7/WT and MCF-7/ADR cells were sus pended in EB" alone, in EB containing 0.5 M NaCl, or in EB containing 0.4 mg/ ml DNase II for 1 h. These suspensions were centrifuged at 1000 x g for 10 min to obtain the extracted nuclear pellets, and these nuclear pellets then were resuspended in buffer containing 1% CHAPS detergent to solubilize remaining nuclear PKC. The detergent-solubilized nuclear fractions were assayed for PKC activity as described in "Materials and Methods" using PKC substrate peptide [(Ser")PKC( 19-31)1 (GIBCO) as phosphoacceptor substrate. The detergent-sol ubilized PKC activities found in the nuclei pretreated as indicated are given as nmol "P incorporated/min/mg protein. Data are expressed as mean ±SEM. Detergent-solubilized Pretreatment with EB" alone were the most resistant with a IC50 of approximately 3 /¿M, while MCF-7/ADRcf cells exhibited a resistance (IC50 -0.3 ,;M) intermediate between MCF-7/ADRjm cells and MCF-7/WT cells (Fig. 4; Table 3). Control, drug-sensitive MCF-7/WT cells exhibited a 1C?,]of 0.04 /UMAdriamycin. The nuclear PKC activity of these three cell lines was deter mined, and the relationship between the nuclear PKC activity and the relative drug resistance was examined (Table 3). The 100 CO _l 90 O 80 LU Ãœ Z 70 CO 60 I- 50 nuclear protein kinase C activity Pretreatment with EB containing 0.5 M NaCl Pretreatment with EB containing DNase II Cell type LLJ MCF-7/WT 0.171 ±0.013 0.106 ±0.023 0.100 ±0.009 Ãœ MCF-7/ADR DC 1.175 ±0.064 1.728 ±0.046 1.244 ±0.059 LU °EB. equilibration buffer [20 mM Tris-Cl (pH 7.5), 2 mvi EDTA, 2 mM EGTA, OL 2 mM dithiothreitol, and protease inhibitors (100 nu ml leupeptin, 50 «<g/ml III aprotinin, 50 *ig/ml (4-amidinophenyl)methanesulfonyl fluoride, 10 »ig/mlsoy bean trypsin inhibitor, and 100 ng/ml pepstatin A)]. ADR |m MCF-7/WT 40 30 20 fractions strongly suggest that the nuclear pool of PKC found in MCF-7/ADR cells is distinct from the cytosol and plasma membrane pools of PKC in these cells. Extraction of PKC from Isolated Nuclei. To determine whether the high level of PKC activity present in the purified nuclear fraction isolated from MCF-7/ADR cells was extractable by high salt concentration or by DNase treatment, the purified nuclei were first washed with 0.5 M NaCl or treated with 0.4 mg/ml DNase II prior to solubilization with detergent (1% CHAPS) (Table 2). Prior extraction of isolated nuclei with buffer containing either 0.5 M NaCl or 0.4 mg/ml DNase II caused approximately a 40 and a 30% decrease, respectively, in LU OC 10 0 0.001 0.01 0.1 1.0 10 100 ADRIAMYCIN Fig. 4. Relative sensitivity of control MCF-7/WT cells and multidrug-resistant MCF-7/ADRcf and MCF-7/ADR'1" cells to the effect of 48-h treatment with increasing concentrations of doxorubicin (Adriamycin). Chemosensitivity of drugsensitive MCF-7/WT cells and multidrug-resistant MCF-7/ADRcf and MCF-7/ ADR'"1 cells to the cytotoxic effect of doxorubicin (Adriamycin) was determined by MTT colorimetrie assay as described in "Materials and Methods." Cells were treated with the indicated concentrations of doxorubicin (Adriamycin) for 48 h. 3754 Downloaded from cancerres.aacrjournals.org on June 17, 2017. © 1992 American Association for Cancer Research. ELEVATED NUCLEAR PKC AND DRUG RESISTANCE trast, MCF-7/ADR cells contained high levels of nuclear PKC activity which eluted in two major peaks from the DEAE column. The first peak eluted with 140 mM NaCl, while the second, more prominent, peak of activity eluted with 180 mM NaCl. Both peaks of nuclear PKC activity noted with the MCFProtein kinase C activity* 7/ADR extract were found to be immunoreactive with both the (nmol 32P incorporated/ pan-specific PKC antiserum and with PKC«-specific antiserum protein)Cell min/mg to by dot blot analysis (data not shown). Adriamycin resistance to type"MCF-7/W (IC,o (MM)0.04 Adriamycinc160600 Western Blot Analysis of PKC in MCF-7/WT and MCF-7/ homogenate0.25 ADR cells. Our finding that the increase in immunohistochem±0.04 ±0.03 MCF-7/ADR'' ical staining and in PKC activity noted in MCF-7/ADR cells is 1.02 ±0.21 0.60 ±0.05 0.25 MCF-7/ADR"" 2.82Relative 1.12 ±0.07 1.37 ±0.08 due to an increase in PKC enzyme was further established by MCF-7(GY)/WT 1.07 ±0.10 0.18 ±0.06 Western blot analysis of total cell extracts using a PKC antibody MCF-7(GY)/W0.3 2.70 ±0.09 1.56 ±0.09 MCF-7(GY)/W10Total 2.68 + 0.12Nucleus0.27 pan-specific for PKC isotypes «,ß,and y (Fig. 6). Markedly 3.1 5 ±0.08Sensitivity " MCF-7/WT. MCF-7/ADRcf. and MCF-7/ADRjm cells were provided by Dr. elevated levels of PKC protein were observed in MCF-7/ADR Kenneth Cowan, National Cancer Institute. NIH. MCF-7(GY)/WT, MCFcells. We also examined the PKC isotype pattern present in the 7(GY)/W0.3, and MCF-7(GY)/W10 cells were provided by Dr. Grace Yen, total cell extracts prepared from these cell lines with a, ß,and National Cancer Institute, NIH. * For PKC activity measurements, histone HI (type III-S) was used as substrate y isotype-specific PKC antibodies (Fig. 6). The antibodies used for MCF-7 cells received from Dr. Kenneth Cowan, and PKC substrate peptide were raised in rabbits against hemocyanin-conjugated synthetic [(Ser")PKC( 19-31)][(Gibco) was used as substrate for the (GY) series of cells. peptides as described in "Materials and Methods." a-isotype ' Data were provided by Dr. Grace Yeh. PKC (PKC«) and 0-isotype PKC (PKC/3) were found to be present in the MCF-7 cell lines. MCF-7/ADR cells contained most resistant cell line (MCF-7/ADRJM1) had the highest nuclear markedly increased amounts of PKCa compared to that of PKC activity among these three cell lines, while the less drugMCF-7/WT cells. No significant difference in the amounts of resistant MCF-7/ADRcl cell line showed an intermediate nu PKC/3 was found in MCF-7/WT cells and MCF-7/ADR cells, clear PKC activity. The control, drug-sensitive MCF-7/WT and we were unable to detect PKC-y in these cell lines. Recently, cells had the lowest nuclear PKC activity. It was of interest to additional members of the PKC family (i.e., 5, t, and f subtypes) note that the level of nuclear PKC activity showed a correlation have been identified (21,22). Preliminary evidence with antisera with the degree of relative drug resistance, while the PKC specific to the <5,e, and f isotypes of PKC indicates that none activities found in the total homogenates were equally elevated of these isoforms is elevated in MCF-7/ADR cells. Because of in the two drug-resistant cell lines (MCF-7/ADRtf and MCFthe different antibody preparations used, it is not possible to 7/ADRjm) compared to that of the drug-sensitive MCF-7/WT Table 3 Relationship between nuclear protein kinase C activity and sensitivity to the cytotoxic effect of Adriamycin in drug-sensitive MCF-7/WT and multidrugresistant MCF- 7/A DR cells Chemosensitivity to the cytotoxic effect of Adriamycin (48-h treatment) was determined by the MTT colorimetrie assay as described in "Materials and Methods." Data are expressed as mean ±SEM. cells. To further establish the relationship between nuclear PKC activity and relative drug resistance in MCF-7 cell lines, we determined the PKC activities of various subcellular fractions prepared from a second set of independently isolated drugsensitive and drug-resistant cells derived from additional MCF7 cell lines [MCF-7(GY) cell lines, kindly provided by Dr. Grace Yeh]. Again, a good correlation was noted between the level of nuclear PKC activity and the relative degree of drug resistance exhibited by these MCF-7(GY) cell lines (Table 3). Although PKC activities associated with the plasma membrane and cytosolic fractions were also somewhat elevated in these drug-resistant cell lines, a close relationship between changes in cytosolic and plasma membrane PKC activity and relative drug resistance was not evident.3 DEAE-Cellulose Chromatography of Nuclear PKC Activity from MCF-7/WT and MCF-7/ADR Cells. To further character ize the elevated nuclear PKC activity found in MCF-7/ADR cells, we have compared the DEAE-cellulose chromatography elution profiles of the detergent-solubilized PKC activities ob tained from nuclei isolated from MCF-7/WT and MCF-7/ADR cells (Fig. 5). The elution profile of nuclear PKC activity from MCF-7/ADR cells was quite different not only quantitatively, but also qualitatively, from that of MCF-7/WT cells. In MCF7/WT cells, the nuclear PKC activity was very low and was mainly eluted from the column with 140 mM NaCl. Since the level of PKC activity present in the nuclear extract from MCF7/WT cells was very low, this observation was confirmed from a similar elution profile obtained by applying three times the amount of nuclear protein on the column (Fig. 5, O). In con ' Unpublished data. so .2 o 70 > E 60 OÕ 50 Si g S 40 Z(N £„ 30 o °"x a -B 20 10 8 12 16 24 0.0 FRACTION NUMBER Fig. 5. DEAE-cellulose column elution profile of protein kinase C activity detergent-solubilized from nuclei isolated from MCF-7/WT and MCF-7/ADR cells. Three hundred ng of nuclear protein detergent-solubilized (1% CHAPS) from nuclei isolated from MCF-7/WT (•)and MCF-7/ADR (A) cells were applied to a 2-ml bed volume DEAE-cellulose column equilibrated with equili bration buffer [20 mM Tris-Cl (pH 7.5), 2 mM EDTA, 2 mM EGTA, 2 mM dithiothreitol. and protein inhibitors (100 ,<u ml leupeptin, 50 Mg/ml apretinen. 50 Mg/ml (4-amidinophenylmethanesulfonyl flouride. 10 ¿igsoybean trypsin in hibitor, and 100 ng/ml pepslatin A)) and eluted with a linear gradient (0-0.5 M) of NaCl in the same buffer. To facilitate better detection of the low levels of nuclear PKC activity present in MCF-7/WT cells, DEAE-cellulose chromatog raphy was also carried out with a 3-fold greater (900 ^g) amount of MCF-7/WT detergent-solubilized nuclear protein (O). Five hundred-fil fractions were col lected, and PKC activity was determined in 20-pl aliquots of each fraction as described in "Materials and Methods." Dotted line, NaCl gradient. 3755 Downloaded from cancerres.aacrjournals.org on June 17, 2017. © 1992 American Association for Cancer Research. ELEVATED NUCLEAR PKC AND DRUG RESISTANCE Pan PKC Aby Anti-PKCa Anti-PKC^ Anti-PKC- 200KFig. 6. Western blot analysis of protein kinase C in total cell extracts of MCF-7/WT and MCF-7/ADR cells. Electrophoresis of total cell extracts prepared from MCF-7/WT and MCF7/ADR cells was carried out on S% SDS-polyacrylamide gel electrophoresis mini-gels as de scribed in "Materials and Methods." Following electrophoresis, proteins were transferred to Immobilon-P membrane. The isotype pattern of PKC was determined by immunoblotting with polyclonal antibodies specific for the a.. '. and y isotypes of PKC. The antibodies used were raised in rabbits against hemocyanin-conjugated syn thetic peptides as described in "Materials and Methods." Protein bands were detected with biotinylated goat anti-rabbit IgG and alkaline phosphatase-conjugated streptavidin. Arrowhead, M, 80.000 PKC band. 116K97K66K- 45K- make a comparison between the amount of PKC«relative to PKQ3 that might be present in the MCF-7 cell lines based upon the intensity of the bands. Results suggest that the pan-specific PKC antisera used recognize the PKC (apparently PKC«)pres ent in the MCF-7/ADR cells to a much greater degree than do the o-specific PKC antisera. Similar immunoblot patterns were observed with purified nuclei isolated from MCF-7/WT and MCF-7/ADR cells (Fig. 7). The purified nuclei isolated from MCF-7/ADR cells again revealed a marked increase in immunostaining with pan-specific PKC antibody and also contained elevated amounts of PKCa. As in the immunoblot analysis of total cell extract, there was little change in PKCß,and we could not detect PKC> in the nuclei prepared from these cell lines (data not shown). When the CHAPS-solubilized nuclear protein fractions were electrophoresed on 10-20% SDS-polyacrylamide gradient gels, electrophoretically transferred to Immobilon-P membranes, and analyzed by immunoblotting with the PKC antiserum panspecific for PKC isotypes a, ß, and 7, the MCF-7/ADR nuclear extract revealed not only a marked increase in immunoreactivity but also a distinct difference in the immunostaining pattern (3 bands) when compared to that of MCF-7/WT cells (2 bands) (Fig. 8). The upper, major PKC band present in nuclei isolated from MCF-7/ADR cells was not detectable in nuclei isolated from MCF-7/WT cells. None of these bands was detected when immunoblots were performed with preimmune serum (data not shown). The reason for the difference in the pattern of PKC bands detected by Western blot analysis of solubili/ed nuclear proteins electrophoresed on 8% polyacrylamide gels (Fig. 7) compared to the immunostaining pattern noted with 10-20% polyacrylamide gradient gels (Fig. 8) is not readily apparent. Nonetheless, this result does further suggest that MCF-7/ADR cells do contain elevated levels of an apparently modified form of nuclear PKC. Pan PKC Aby Anti-PKC« 116K97K66K- 45K- Fig. 7. Western blot analysis of protein kinase C in nuclei isolated from MCF7/WT and MCF-7/ADR cells. The purified nuclear pellet obtained from MCF7/WT and MCF-7/ADR cells was solubilized in boiling SDS-polyacrylamide gel electrophoresis sample buffer and drawn through a 23-gauge needle to shear DNA. Equivalent amounts (10 ^g) of nuclear protein were electrophoresed by 8% SDS-polyacrylamide gel electrophoresis and transferred to Immobilon-P mem brane. PKC proteins were detected by probing the blot with rabbit anti-peptide antiserum pan-specific for i>,/i, and y isotypes of PKC, or with rabbit anti-peptide antiserum specific for a isotype of PKC only. Arrowhead, position of PKC band. 3756 Downloaded from cancerres.aacrjournals.org on June 17, 2017. © 1992 American Association for Cancer Research. ELEVATED NUCLEAR PKC AND DRUG RESISTANCE 97.4K66.5K - PKC 45.7K - 31.0K21.5K14.4K- Fig. 8. Western blot analysis of PKC immunoreactive proteins extracted from nuclei isolated from MCF-7/WT and MCF-7/ADR cells and electrophoresed using 10-20% SDS-polyacrylamide gradient gels. Detergent (1% CHAPS)-solubilized protein fractions were prepared from purified nuclei isolated from MCF7/WT and MCF-7/ADR cells, and the solubilized protein fractions were dena tured in SDS sample buffer. Equivalent amounts (2 ng) of SDS denatured nuclear protein were electrophoresed using 10-20% SDS-polyacrylamide gradient gels and the protein bands then were electrophoretically transferred to Immobilon-P membranes. PKC immunoreactive proteins were detected by probing the blot with rabbit anti-peptide antiserum pan-specific for a, ß,and y isotypes of PKC. DISCUSSION Several lines of evidence indicate the possible involvement of PKC in modulating cellular resistance to antitumor drugs of the natural products class. Protein kinase C activity has been reported to be elevated in several cell types exhibiting the multidrug-resistance phenotype, including MCF-7 human breast carcinoma cells (14), HL-60 leukemia cells (23, 24), murine fibrosarcoma cells (15), and KB human carcinoma cells (25). Results of cell growth and cytotoxicity studies in which PKC activity has been altered with TP A and with the H-7 [1(5-isoquinolinesulfonyl)-2-methylpiperazine] inhibitor also in dicate a close relationship between PKC activity and cell sen sitivity to the cytotoxic effects of these anticancer drugs (1417). Furthermore, studies suggest that PKC-activation may be involved in the phosphorylation of the membrane P-glycoprotein involved in drug transport. Enhanced phosphorylation of P-glycoprotein has been noted with phorbol ester treatment of K562/ADM cells (26), and Chambers et al. (25) have reported that the P-glycoprotein from drug-resistant human KB carci noma cells can serve as a good substrate for PKC in vitro. In this study, we have presented a further characterization of the enhanced levels of PKC found in multidrug-resistant MCF7 human breast carcinoma cells when compared to that of control drug-sensitive MCF-7/WT cells. Our results indicate that MCF-7/ADR drug-resistant cells contain markedly ele vated levels of nuclear PKC. This conclusion is based upon the following data: (a) immunohistochemical localization studies using a PKC antibody pan-specific for a, ß,and 7 isotypes of PKC indicated that immunoreactivity was enhanced in MCF7/ADR cells compared to that of MCF-7/WT cells, with pro nounced staining noted in the nuclear region; (b) other immu nohistochemical studies with nuclei isolated from MCF-7 cells also revealed a significant increase in the intensity of iminium staining for PKC in nuclei isolated from MCF-7/WT cells; (c) MCF-7/ADR cells contained a markedly elevated level of nu clear PKC activity compared to that of MCF-7/WT cells; (d) another set of multidrug-resistant MCF-7 cell lines [MCF7(GY)/W0.3 and MCF-7(GY)/W10 cells] also was found to exhibit an increased level of nuclear PKC activity compared to that of control drug-sensitive MCF-7(G Y)/WT cells; (e) West ern blot analysis of total cell extracts with a pan-specific PKC antibody recognizing the a, 0, and y isotypes of PKC, as well as with a, ß,and 7 isotype-specific PKC antibodies, showed that MCF-7/ADR cells contained highly elevated amounts of PKC«when compared to that of MCF-7/WT cells, while no difference was observed in the amounts of PKC/i, and PKC7 was not detected in these cell lines; and (/) immunoblot analysis of proteins extracted from purified nuclei isolated from these MCF-7 cell lines revealed that nuclei isolated from MCF-7/ ADR cells contained highly elevated levels of PKCa. Protein kinase C has been found to be associated with the nuclear fraction of a number of cell types (18, 27-32). To establish that the markedly elevated level of PKC found in MCF-7/ADR cells represents a pool of PKC separate from the cytosolic and plasma membrane fractions of PKC, we carried out time course studies of changes in the distribution and downregulation of PKC activity in the different subcellular fractions induced by treatment of these cells with 1 ^M TPA (Fig. 3). When PKC activity had been down-regulated by prolonged (24h) exposure of MCF-7/ADR cells to TPA, significant PKC activity still remained in both the cytosolic (20% of control) and plasma membrane (40% of control) subcellular fractions. In contrast, there was a complete loss of the nuclear pool of PKC activity within 12 h after exposure of the cells to TPA. This, along with the differences noted in the rapid (30-min) redistribution of PKC from the cytosolic to the plasma mem brane fraction, compared with no change detected in the nuclear PKC activity at 30 and 90 min of TPA treatment, strongly suggests that the nuclear pool of PKC present in MCF-7/ADR cells is distinct from the cytosolic and plasma membrane pools of PKC. There appears to be a good correlation between the level of nuclear PKC activity found and the degree of drug resistance in MCF-7 human breast carcinoma cells. This was observed in two different MCF-7 cell lines: (a) MCF-7 cells obtained from Dr. Kenneth Cowan [control MCF-7/WT cells and multidrugresistant MCF-7/ADR cells (MCF-7/ADR'1 and MCF-7/ ADRjm)]; (b) MCF-7 cells obtained from Dr. Grace Yeh [control MCF-7(GY)/WT cells and multidrug-resistant MCF-7(GY)/ W0.3 cells and MCF-7(GY)/W10 cells]. In both MCF-7 cell lines the most resistant cell types were found to contain the highest level of nuclear PKC activity, with less resistant cell types having intermediate levels of nuclear PKC activity relative to the very low level of nuclear PKC activity found in the control, drug-sensitive cell types. It should be noted that this quantitative correlation between the level of nuclear PKC and 3757 Downloaded from cancerres.aacrjournals.org on June 17, 2017. © 1992 American Association for Cancer Research. ELEVATED NUCLEAR PKC AND DRUG RESISTANCE the degree of drug resistance is masked when PKC activities determined in total cell homogenates are compared. A close relationship between the level of PKC activity present in the cytosol and plasma membrane and the degree of drug resistance was not evident. Observations made during the course of this investigation indicate that the PKC activities found in the purified nuclear fractions isolated from MCF-7/WT and MCF-7/ADR cells exhibit somewhat different characteristics, suggesting that a different or modified form of PKC may be present in the nucleus of MCF-7/ADR cells, (a) The DEAE-cellulose column elution profiles of nuclear PKC isolated from MCF-7/WT and MCF7/ADR cells revealed both quantitative and qualitative differ ences between these two activities (Fig. 5). With MCF-7/WT cells, the low amount of nuclear PKC activity detected was eluted with 140 ITIMNaCl. In contrast, two major peaks of nuclear PKC activity were observed with the extract from MCF7/ADR cells. The first peak eluted at 140 HIMNaCl, while the second, more prominent, peak of MCF-7/ADR nuclear PKC activity eluted at 180 HIMNaCl. The explanation for the altered elution profile for the second, high salt peak of PKC activity noted with the nuclear protein fraction isolated from MCF-7/ ADR cells remains to be established. Both changes in the phosphorylation state of PKC (33) and changes in the oxidative state of PKC (34, 35) have been reported to modify PKC to a form which élûtes from DEAE-cellulose at higher (200-250 HIM) salt concentrations. Modifications of this type may be responsible for the altered form of PKC found in nuclei isolated from MCF-7/ADR cells, and these possibilities are currently under investigation, (b) When the CHAPS-solubilized nuclear protein fractions of MCF-7/WT and MCF-7/ADR cells were electrophoresed using 10-20% polyacrylamide gradient gels, Western blot analysis revealed a distinct difference in both the intensity and pattern of PKC immunostaining between the two cell types (Fig. 8). The upper, highly stained PKC band found in the nuclear extract of MCF-7/ADR cells was not detectable in the nuclear fraction of MCF-7/WT cells, (c) Our preliminary data indicate that the nuclear pool of PKC present in MCF-7/ ADR cells is rapidly inactivated in response to treatment of these multidrug-resistant cells with H2O2 and with sangivamycin. Neither of these treatments results in a loss of PKC activity from the nuclear fraction of drug-sensitive MCF-7/WT cells, further indicating a difference in these nuclear PKC pools.4 In conclusion, the results presented indicate that drug-resist ant MCF-7/ADR cells contain not only increased amounts of cytosolic PKC but also markedly elevated levels of an appar ently modified form of PKCa at the nucleus. However, it remains to be determined what role this increase in nuclear PKC might play in the development and modulation of multidrug resistance. Nuclear proteins such as histone H1 (36), DNA methyltransferase (37), topoisomerase II (38), poly(ADP-ribose) synthetase (39), DNA polymerase a (40), and lamin B (41) all have been tentatively identified as possible substrates for phosphorylation by PKC. In addition, studies have been reported which support a role for PKC in the regulation of selected gene expression (42-44). Thus, it is conceivable that the elevated cytosolic PKC noted in drug-resistant MCF-7/ ADR cells may catalyze the phosphorylation of proteins such as the gpl70 transporter protein (25, 26) to mediate drug resistance, while the nuclear pool of PKC found in MCF-7/ ADR cells may play a role in altering the expression of gene(s) ' Manuscripts in preparation. important for either the onset or the maintenance of the drugresistant state of these cells. ACKNOWLEDGMENTS We are grateful to Dr. Kenneth Cowan (National Cancer Institute, NIH, Bethesda, MD) for generously providing us MCF-7/WT cells and MCF-7/ADR cells, and to Dr. Grace Yeh (National Cancer Institute) for kindly providing us MCF-7(GY)/WT cells, MCF-7(GY)/W0.3, and MCF-7(GY)/W 10 cells. We also wish to thank Dr. Ulf Rapp (National Cancer Institute, Frederick County Research Facility, NIH, Frederick, MD) and Dr. Peter Blumberg (National Cancer Institute) for help and advice in the preparation and characterization of the PKC antisera used in this study. REFERENCES 1. Baker, R. M., and Ling. V. Membrane mutants of mammalian cells in culture. In: E. Korn (ed.). Methods in Membrane Biology, Vol. 9, pp. 337384. New York: Plenum Publishing Corp., 1978. 2. Biedler, J. L., and Peterson, R. H. F. Altered plasma membrane glycoconjugates of Chinese hamster cells with acquired resistance to actinomycin D, daunorubicin, and vincristine. In: A. C. Sartorelli, J. S. Lazo, and J. R. Berlino (eds.). Molecular Actions and Targets for Cancer Chemotherapeutic Agents, pp. 453-476. New York: Academic Press. 1981. 3. Bradley, G.. Naik, M., and Ling. V. P-Glycoprotein expression in multidrugresistant human ovarian carcinoma cell lines. Cancer Res., 49: 2790-2796, 1989. 4. Endicott, J. A., and Ling, V. The biochemistry of P-glycoprotein-mediated multidrug resistance. Annu. Rev. Biochem., 58: 137-171, 1989. 5. Pastan, I., and Gottesman, M. Multiple-drug resistance in human cancer. N. Engl. J. Med., 316: 1388-1393, 1987. 6. Gottesman, M. M.. and Pastan, I. The multidrug transporter, a double-edged sword. J. Biol. Chem.. 263: 12163-12166, 1988. 7. Greenberger, L. M., Lothstein, L., Williams, S. S., and Horrwitz, S. B. Distinct P-glycoprotein precursors are overproduced in independently iso lated drug-resistant cell lines. Proc. Nati. Acad. Sci. USA, 85: 3762-3766, 1988. 8. Somfai-Relle, S., Suzukake, K., Vistica, B. P., and Vistica, D. T. Reduction in cellular glutathione by buthionine sulfoximine and sensitization of murine tumor cells resistant to i -phenylalanine mustard. Biochem. Pharmacol., .M: 485-490, 1984. 9. Hamilton, T. C.. Winkler. M. A.. Louie. K. G., Batist, G., Behrens, B., Tsuruo, T., Grotzinger, K. R., Mckoy, W. M., Young, R. C., and Ozols, R. F. Augmentation of Adriamycin. melphalan and cisplatin cytotoxicity in drug-resistant and -sensitive human ovarian cancer cell lines by buthionine sulfoximine-mediated glutathione depletion. Biochem. Pharmacol., 34: 2583-2589,1985. 10. Batist, G., Tulpule, A.. Sinha. B. K., Kathi, A. G., Myers, C. E., and Cowan, K. H. Overexpression of a novel anionic glutathione transferase in multidrugresistant human breast cancer cells. J. Biol. Chem., 261:15544-15549, 1986. 11. Cowan, K. H., Batist, G.. Tulpule, A., Sinha, B. K., and Myers, C. E. Similar biochemical changes associated with multidrug resistance in human breast cancer cells and carcinogen-induced resistance to xenobiotics in rats. Proc. Nati. Acad. Sci. USA, 83:9328-9332, 1986. 12. Mungikar, A., Chitnis, M., and Gothoskar, B. Mixed function oxidase enzymes in Adriamycin-sensitive and -resistant sublines of P388 leukemia. Chem.-Biol. Interact., 35: 119-124, 1981. 13. Mimnaugh, E. G.. Dusre, L.. Atwell, J., and Myers, C. E. Differential oxygen radical susceptibility of Adriamycin-sensitive and -resistant MCF-7 human breast tumor cells. Cancer Res., 49: 8-15, 1989. 14. Fine, R. L., Palei, J., and Chabner, B. A. Phorbol esters induce multidrug resistance in human breast cancer cells. Proc. Nati. Acad. Sci. USA, 85:582586, 1988. 15. O'Brian, C. A., Fan, D., Ward. N. E.. Seid, C, and Fidler, I. J. Level of 16. 17. 18. 19. 20. protein kinase C activity correlates directly with resistance to Adriamycin in murine fibrosarcoma cells. FEBS Lett., 246: 78-82, 1989. Ferguson, P. J.. and Cheng, Y-C. Transient protection of cultured human cells against antitumor agents by 12-O-tetradecanoyl-13-acetate. Cancer Res., Â¥7:433-441, 1987. Kessel, D. Effects of phorbol esters and doxorubicin transport systems. Biochem. Pharmacol., 37: 2297-2299, 1987. Thomas, T. P., Talwar, H. S., and Anderson, W. B. Phorbol ester-mediated association of protein kinase C to the nuclear fraction in NIH 3T3 cells. Cancer Res., 48: 1910-1919. 1988. Mitchell, F. E., Marais, R. M., and Parker, P. J. The phosphorylation of protein kinase C as a measure of activation. Biochem. J., 261: 131-136, 1989. Bradford, M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing principle of protein-dye binding. Anal. Biochem., 72: 248-254, 1976. 3758 Downloaded from cancerres.aacrjournals.org on June 17, 2017. © 1992 American Association for Cancer Research. ELEVATED NUCLEAR PKC AND DRUG RESISTANCE 21. Ono, Y., Fuji!, T., Ogita, K., Kikkawa, U., Igarashi, K., and Nishizuka, Y. The structure, expression, and properties of additional members of the protein kinase C family. J. Biol. Chem., 263:6927-6932, 1988. 22. Schaap, IX, and Parker, P. J. Expression, purification, and characterization of protein kinase C-«.J. Biol. Chem., 265: 7301-7307, 1990. 23. Aquino, A., Hartman, K. D., Knode, M. C., Grant, S., Huang, K. P., Niu, C. II., and Glazer, R. I. Role of protein kinase C in phosphorylation of vinulin in Adrianiu-in- resistant HL-60 leukemia cells. Cancer Res., -IK:33243329, 1988. 24. Aquino, A., Warren, B. S., Omichinski, J., Hartman, K. D., and Glazer, R. I. Protein kinase Cy is present in Adriamycin resistant HL-60 leukemia cells. Biochem. Biophys. Res. Commun., 166: 723-728, 1990. 25. Chambers, T. C., McAvoy, E. M., Jacobs, J. W., and Eilon, G. Protein kinase C phosphor) lates P-glycoprotein in multidrug resistant human KB carcinoma cells. J. Biol. Chem., 265: 7679-7687, 1990. 26. Hamada, II.. Hagiwara, K. I., Nakajima, T., and Tsuruo, T. Phosphorylation of the M, 170,000 to 180,000 glycoprotein specific to multidrug-resistant tumor cells: effects of verapamil, trifluoperazine, and phorbol esters. Cancer Res., 47: 2860-2865, 1987. 27. Mason-Garcia, M., Weill, C., and Beckman, B. S. Rapid activation by erythropoietin of protein kinase C in nuclei of erythroid progenitor cells. Biochem. Biophys. Res. Commun., 168: 490-497, 1990. 28. Masmoudi, A., Labourdette, G., Mersel, M., Huang, F. I... Huang, K-l'.. Vincendon, G., and Malviya, A. N. Protein kinase C located in rat liver nuclei. J. Biol. Chem., 264: 1172-1179, 1989. 29. Capitani, S., Girard, P. R., Mazzei, G. J., Kuo, J. F., Berezney, R., and Manzoli, F. A. Immunochemical characterization of protein kinase C in rat liver nuclei and subcellular fractions. Biochem. Biophys. Res. Commun., 142: 367-375, 1987. 30. Chen, Z. Z., McGuire, J. C., Leach, K. L., and Cambiar, J. C. Transmem brane signalling through B cell Mill class II molecules: anti-la antibodies induce protein kinase C translocation to the nuclear fraction. J. Immunol., 138: 2345-2352, 1987. 31. Fields, A. P., Pettit, G. R., and May, W. S. Phosphorylation of lamin B at the nuclear membrane by activated protein kinase C. J. Biol. Chem., 263: 8253-8260,1988. 32. Leach, K. L., Powers, E. A., Ruff, V. A., Jaken, S., and Kaufmann, S. Type 3 protein kinase C localization to the nuclear envelope of phorbol ester- treated NIH 3T3 cells. J. Cell Biol., 109: 685-695, 1989. 33. Molina, C. A., Weyman, C. M., and Ashendel, C. L. Regulation of protein kinase C via phosphorylation. Proc. Am. Assoc. Cancer Res., 31:149, 1990. 34. Gopalakrishna, R., and Anderson, W. B. ( a •¿ and phospholipid-independent activation of protein kinase C by selective oxidative modification of the regulatory domain. Proc. Nati. Acad. Sci. USA, 86:6758-6762, 1989. 35. Gopalakrishna, R., and Anderson, W. B. Reversible oxidative activation and inactivation of protein kinase C by the mitogen/tumor promoter periodate. Arch. Biochem. Biophys., 285: 382-387, 1991. 36. Iwasa, Y., Takai, Y., Kikkawa, U., and Nishizuka, Y. Phosphorylation of calf thymus HI histone by calcium-activated, phospholipid-dependent protein kinase. Biochem. Biophys. Res. Commun., 96: 180-187, 1985. 37. Roach, A. D., Roach, P. J., Zucker, K. E., and Smith, S. S. Selective phosphorylation of human DNA methyl transferase by protein kinase C. FEBS Lett., 197: 149-153, 1986. 38. Sahyoun, N., Wolf, M., Besterman, J., Hsieh, T-S., Sander, M., LeVine, H., Chang, K-J., and Cuatrecasas, P. Protein kinase C phosphorylates topoisomerase II: topoisomerase activation and its possible role in phorbol esterinduced differentiation of HL-60 cells. Proc. Nati. Acad. Sci. USA, 83:16031607, 1986. 39. Tanaka, Y., Koide, S. S., Yoshihara, K., and Kamiya, T. Poly(ADP-ribose)synthetase is phosphorylated by protein kinase C in vitro. Biochem. Biophys. Res. Commun.. 148: 709-717, 1987. 40. Krauss, S. W., Mochly-Rosen, D., Koshland, D. E., Jr., and Linn, S. Expo sure of I lei .a DNA polymerase a to protein kinase C affects its catalytic properties. J. Biol. Chem., 262: 3432-3435, 1987. 41. Fields, A. P., Pettit, G. R., and May, W. S., Jr. Phosphorylation of lamin B at the nuclear membrane by activated protein kinase C. J. Biol. Chem., 263: 8253-8260,1988. 42. Jetten, A. M., Ganong. B. R.. Vandenbark, G. R., Shirley, J. E., and Bell, R. M. Role of protein kinase C in diacylglycerol-mediated induction of ornithine decarboxylase and reduction of epidermal growth factor receptor. Proc. Nati. Acad. Sci. USA, 82: 1941-1945, 1985. 43. Imagawa, M., Chiù,R., and Karin, M. Transcription factor AP-2 mediates induction by two different signal transduction pathways: protein kinase C and CAMP. Cell, 51: 251-260, 1987. 44. McCaffrey, P., Ran, W., Campisi, J., and Rosner, M. R. Two independent growth factor-generated signals regulate c-fos and c-myc mRNA levels in Swiss 3T3 cells. J. Biol. Chem., 262: 1442-1445, 1987. 3759 Downloaded from cancerres.aacrjournals.org on June 17, 2017. © 1992 American Association for Cancer Research. Elevated Level of Nuclear Protein Kinase C in Multidrug-resistant MCF-7 Human Breast Carcinoma Cells Sung Ae Lee, James W. Karaszkiewicz and Wayne B. Anderson Cancer Res 1992;52:3750-3759. Updated version E-mail alerts Reprints and Subscriptions Permissions Access the most recent version of this article at: http://cancerres.aacrjournals.org/content/52/13/3750 Sign up to receive free email-alerts related to this article or journal. To order reprints of this article or to subscribe to the journal, contact the AACR Publications Department at [email protected]. To request permission to re-use all or part of this article, contact the AACR Publications Department at [email protected]. Downloaded from cancerres.aacrjournals.org on June 17, 2017. © 1992 American Association for Cancer Research.