* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Succinate
Mitochondrion wikipedia , lookup
Photosynthesis wikipedia , lookup
Biochemistry wikipedia , lookup
Adenosine triphosphate wikipedia , lookup
Nicotinamide adenine dinucleotide wikipedia , lookup
Microbial metabolism wikipedia , lookup
Citric acid cycle wikipedia , lookup
Photosynthetic reaction centre wikipedia , lookup
Light-dependent reactions wikipedia , lookup
NADH:ubiquinone oxidoreductase (H+-translocating) wikipedia , lookup
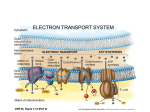
CHAPTER 17 - ELECTRON TRANSPORT AND OXIDATIVE PHOSPHORYLATION Introduction - The complete aerobic catabolism of glucose can be represented as: C6H12O6 + 6 O2 6 6 CO2 + 6 H2O - The reactions of glycolysis and the citric acid cycle have resulted in the degradation of glucose to CO2, but the electrons have been transferred to the redox coenzymes NAD+ and FAD: Glucose 2 Glyceraldehyde 3-phosphate 2 1,3-bisphosphoglyerate 2 2 NAD+ NADH Pyruvate NAD+ 2 2 NADH Acetyl CoA Citrate Oxaloacetate 2 NADH Isocitrate 2 NAD+ 2 NAD+ 2 NADH Alpha ketoglutarate Malate Succinate 2 NADH Succinyl CoA Fumarate 2 FADH2 Succinate 2 FAD 2 NAD+ C6H12O6 + 10 NAD+ + 2 FAD 6 10 NADH + 2 FADH2 + 6 CO2 - We now need to study how these reduced electron carriers transport their electrons through the mitochondrial electron transport system (electron transport) to the ultimate electron acceptor, O2, thereby generating sufficient energy to synthesize ATP (oxidative phosphorylation). - Since the transfer of electrons occurs with a positive DeltaE, the resulting free energy change will be negative (DeltaG - nFDeltaE). This free energy is stored in a proton gradient which is then used to drive a membrane-bound ATP-ase in reverse (chemiosmotic principle): H+ Out NADH O2 Electron transport system + NAD H2O FADH2 FAD In ADP + Pi H+ ATP H+ - The overall DeltaE0' for electron transport can be determined from the standard reduction potentials for NAD+ and O2: - (NAD+ + H+ + 2 e- 6 NADH, DeltaE0' = -0.315 Volts) ½ O2 + 2 H+ + 2 e- 6 H2 O, DeltaE0' = 0.815 Volts NADH + ½ O2 + H+ 6 H2O + NADH, DeltaE0' = -(-0.315 V) + 0.815 V = 1.130 V DeltaG0' = -nF DeltaE0' = -(2)(96,485 J/(V mole))(1.130 V) = -218,000 J/ mole = - 218kJ/mole - Electron transport and oxidative phosphorylation take place in the mitochondria. See text, pp, 493-4 for description (Inner and outer membranes, matrix, cristae). Mitochondrial Electron Transport - Note that since electron transport takes place in the mitochondria, the NADH produced during glycolysis must be transported into the matrix. Since the inner membrane is impermeable to NADH only the reducing power of NADH can enter, via either the malate (Figure 15-27) or glycerol phosphate (Figure 17-4) shuttles. In the latter case cytosolic NADH is exchanged for FADH2 which, as we will see, results in the formation of fewer ATP than is the case for NADH. Note from Table 13- 3, p. 373, that the standard reduction potential for FAD is less negative than for NAD+ (-0.219 vs. -0.315 V). This means that NADH has a more positive oxidation potential and hence is a better reducing agent than FADH2, thus yields more energy when it coughs up its electrons, resulting in greater ATP production. Types of electron transport components: - Cytochromes contain heme groups (like hemoglobin and myoglobin), whose Fe atoms reversibly cycle between the ferrous (+2) and ferric (+3) states. See p. 505 - Fe-S proteins also contain Fe which cycles between the +2 and +3 states. In these proteins Fe is coordinated to either organic (cysteine ) or elemental sulfur. See Figure 17-9 for a picture of an iron-sulfur cluster, even though this figure depicts ferredoxin, a bacterial protein. - Flavin (FMN)-based dehydrogenase - Coenzyme Q (CoQ) H O OH H3 CO + CH3 H3 CO H (H2C CH C CH2 )10 H H3 CO CH3 H3 CO O (H2C CH C CH2 )10H OH Reduced Oxidized Note that CoQ is the only non-protein component of the electron transport chain. A two-electron transfer is depicted above. The long isoprenoid tail confers lipid solubility to CoQ. The reduced form is capable of losing a single H atom (H+ + e-) to form a semiquinone radical: OH O H3 CO H3 CO CH3 H3 CO H2C ( CH C CH2 OH -H -e )10 H CH3 H3 CO H2C ( CH C CH2 OH Note also that since components of the electron transport chain are for the most part either one )10H (cytochromes) or two (NADH, FADH2) electron carriers, the ability of CoQ to function as either a oneelectron or two-electron carrier allows it to act as a “go-between” when passing electrons from the twoelectron carriers and one-electron carriers (see, for example, Figure 17-13). - The sequence of electron transport components is governed by the necessity that each transfer occur spontaneously, i.e., with a positive DeltaE (and - DeltaG). We have already seen that when determining the direction of spontaneous electron transfer between two components whose half cell standard reduction potentials are available (see Table 13-3, p. 373), the electron donor is the component whose standard reduction potential is the more negative, or the less positive, of the two. Thus, for example, ubiquinol will donate electrons spontaneously (under standard conditions) to cyt b which will in turn donate spontaneously to cyt c1, and so on. - These various components are arranged into 4 large, immobile complexes, labeled complex I through complex IV (see Figure 17-8). Note that various respiratory inhibitors block the flow of electrons at various points (see Figure 17-7). These inhibitors include rotenone, a plant toxin, amytal, a barbiturate antimycin A and cyanide. Note also that CoQ and cytochrome c are mobile carriers which shuttle electrons from complexes I and II to complex III and from complex III to complex IV, respectively. Cytochrome c is the only water soluble component of the electron chain. Cytochrome c, present in all organisms with mitochondrial electron transport chains, evolved more than 1.5 billion years ago before the divergence of plants and animals. Its function has been preserved throughout this period. Cytochrome c of any eukaryotic species interacts in vivo with complex IV of any other species tested thus far, and the reduction potential of all cytochrome c’s is about +0.25 V. Evidently, nature stumbled on a very efficient structure/function relationship early in the evolutionary development of cytochrome c and stuck with it. ATP Synthesis - By use of Table 13-3, the potential drops across complexes I, III and IV correspond to sufficiently large and negative free energy changes to make ATP (see Figure 17-7). NADH is a strong enough reducing agent to pass electrons into the electron transport chain from complexes I to III to IV. Since FADH2 is not a powerful enough reducing agent to pass electrons through complex I and must therefore pass electrons in at complex III, bypassing complex I. From an energetic viewpoint, this is why NADH is “worth” 3 ATP, whereas FADH2 is only worth 2. - Recall that substrate-level phosphorylations involved high-energy intermediates, such as 1,3bisphosphoglycerate and succinyl CoA. Despite attempts to isolate similar high-energy intermediates involved in oxidative phosphorylation, none were ever found. Peter Mitchell proposed the chemiosmotic theory in 1961, and this was eventually accepted. According to this theory, the free energy of electron transport is conserved by pumping protons (H+) from the mitochondrial matrix to the intermembrane space (cytosolic side of inner membrane) to create an electrochemical proton gradient across the inner mitochondrial membrane. Protons then flow back down this gradient through a membrane-bound ATP-ase (ATP synthase), the free energy released driving the ATP-ase in reverse, i.e., in the direction of ATP synthesis (see Figure 17-18). - Although somewhat esoteric, this theory seems more plausible when it is noted that some of the electron-transport components give off protons when oxidized: NADH 6 NAD+ + 2e- + H+ CoQH2 6 CoQ + 2 e- + 2 H+ - A favorable membrane potential, (DeltaØ = 0.168V, negative on the inside) also favors proton flow back into the matrix. This potential is partly due to the fact that the inner leaflet of the membrane contains acidic phospholipids such as phosphatidyl serine and phosphatidyl ethanolamine, whose head groups have a formal negative charge, and partly due to the fact that the inner mitochondrial membrane is impermeable to small ions such as Cl -, which thus cannot equalize the charge imbalance due to proton pumping. The pH difference across the inner mitochondrial membrane has been measured at 0.75 pH units. This corresponds to the following free energy change: DeltaG = DeltaG0' + RT ln Q + ZF DeltaØ = 2.3 RT log Q + DeltaØ = 2.3RT(log[H+]in - log[H+]out) + DeltaØ = 2.3RT(pHout - pHin) + DeltaØ = 2.3(8.3145 J/mole)(310 K)(-0.75) + (1)(96485 J/(mole V)(-0.168V) = -4.45 kJ - 16.21 kJ = -20.7 kJ per mole of protons. - Since the estimated DeltaG required for ATP synthesis (from ADP) is +40 - +50 kJ/mole under physiological conditions, at least two moles of protons must be pumped back inside to provide enough free energy to synthesize 1 mole of ATP. - ATP synthase is a multi-subunit transmembrane protein consisting of two functional units, Fo (o for oligomycin, which blocks proton flow through the channel) and F1. Fo is the water-insoluble membrane channel through which protons flow. F1 is a water-soluble peripheral protein which forms “knobs” which protrude into the matrix and which contain the ATP-ase activity (see Figure 17-19). Recall that no high-energy intermediates such as PEP are known to be involved in oxidative phosphorylation. Instead, available evidence supports the so-called binding charge mechanism, according to which flow of protons through Fo down a concentration gradient provides free energy which is transmitted to F1 in the form of conformational changes which result in the synthesis of ATP. - The existence of uncoupling agents, such as 2,4-dinitrophenol, which uncouple electron transport from oxidative phosphorylation, lends support to the chemiosmotic theory (see Figure 17-22). Control of Oxidative Phosphorylation - The cytochrome reductase (system IV) reaction is the only component of the electron transport chain that is noticeably exergonic. Electron exchange between components “upstream” of system IV, or from NADH to cyt c, are close to equilibrium (DeltaG0' -0): 1/2 NADH + cytochrome c (Fe+3 ) + ADP + P i + Keq = [NAD ] [NADH] 1/2 NAD+ + cytochrome c (Fe+2 ) + ATP 1/2 [cyt c(Fe+2)] [ATP] [cyt c)Fe )] [ADP][Pi} +3 Since cytochrome reductase receives electrons from cyt c (Fe+2), the above equilibrium expression can be rearranged to express the availability of substrate (i.e., electrons from cyt c (Fe+2)) from points “upstream”: [cyt c(Fe +2)] +3 = Keq [cyt c)Fe )] [NADH] + [NAD ] 1/2 [ADP][P i} [ATP] The higher this ratio, the greater the flow of electrons through system IV, and vice versa. In times of energy need (low [ATP], high [ADP]), electron flow can thus be seen to increase through system IV, thereby increasing ATP production. Similarly, high [NADH], or reducing power, also increases electron flow. - Since glycolysis and the citric acid cycle supply the reducing power (NADH and FADH2) to feed electrons through electron transport, an adequate supply of electrons for electron transport is provided by regulation of the control points of glycolysis and the citric acid cycle. We know that high energy has an inhibitory effect on these pathways (high [ATP] inhibits glycolysis and also the citric acid cycle to a certain extent (ATP inhibits á-ketoglutarate dehydrogenase); high [NADH] inhibits the citric acid cycle (ATP is an allosteric inhibitor, NADH acts by product inhibition). Thus, ATP and NADH ultimately inhibit the flow of electrons through electron transport, which subsequently reduces the rate of oxidative phosphorylatio (ATP production). See Figure 17-23 for a summary of this coordinated control. Note that from the above discussion NADH is seen both to increase the flow of electrons through system IV, and to inhibit the citric acid cycle. This is not as inconsistent as it seems. NADH provides a direct source of electrons for electron transport, hence will increase the rate of electron transport. At the same time, by inhibiting the citric acid cycle, it inhibits further production of NADH. Problems: 2, 3, 4 (see Figure 17-7 for the effects of amytal on electron transport), 7, 9