* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Examples
Drug discovery wikipedia , lookup
Catalytic triad wikipedia , lookup
Gaseous signaling molecules wikipedia , lookup
Isotopic labeling wikipedia , lookup
Deoxyribozyme wikipedia , lookup
Photosynthesis wikipedia , lookup
Fatty acid synthesis wikipedia , lookup
Microbial metabolism wikipedia , lookup
Fatty acid metabolism wikipedia , lookup
Genetic code wikipedia , lookup
Peptide synthesis wikipedia , lookup
Nucleic acid analogue wikipedia , lookup
Protein structure prediction wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Amino acid synthesis wikipedia , lookup
Metalloprotein wikipedia , lookup
Proteolysis wikipedia , lookup
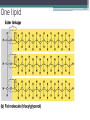
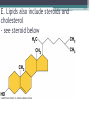
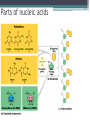
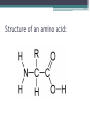
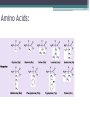
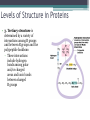
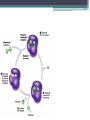
Topic 2 Biochemistry I. Biologically Important Compounds • A. Organic and Inorganic Compounds ▫ 1. organic compounds – contain the element carbon – also contain hydrogen Examples: ▫ 2. inorganic compounds – any compound that is not organic Examples: II. Carbohydrates and Lipids • A. Carbohydrates – compounds of carbon, hydrogen, and oxygen – have a ratio of 2 hydrogen to 1 oxygen ▫ 1. monosaccharides – simple sugars – glucose is the most common simple sugar – other simple sugars include galactose and fructose Glucose ▫ 2. disaccharides – sugars whose molecules are made up of two monosaccharide molecules bonded together – maltose and sucrose are two examples Disaccharide 3. polysaccharides – complex carbohydrates made up of chains of monosaccharides – starch, cellulose and glycogen are examples – starch is a food storage compound in plants, cellulose makes up the cell walls of plants, glycogen is a food storage compound in animals Polysaccharide • B. Dehydration Synthesis – chemical process by which simple molecules are joined to form larger molecules with the removal of water • C. Hydrolysis – chemical process by which larger molecules are broken down by the addition of water – opposite of dehydration synthesis • D. Lipids – serve mainly as sources of energy and structures for cell membranes – include fats(solids from animals), oils(liquids from plants), and waxes – the ratio of hydrogens to oxygens is greater than 2 : 1 and varies from one lipid to another – made from one glycerol molecule and three fatty acids by dehydration synthesis Lipids – one glycerol and three fatty acids One lipid E. Lipids also include steroids and cholesterol - see steroid below III. Nucleic Acids and Proteins • A. Nucleic Acids - long chains of repeating units that carry hereditary or genetic information and control the life processes of cells ▫ 1. DNA – deoxyribonucleic acid – genetic material ▫ 2. RNA – ribonucleic acid – controls production of proteins • B. Nucleic acids are made of chains of repeating units called nucleotides – nucleotides have three parts: ▫ 1. a 5 carbon sugar – DNA has deoxyribose and RNA has ribose ▫ 2. a phosphate group ▫ 3. a nitrogenous base – DNA has adenine, thymine, guanine and cytosine – RNA has adenine, uracil, guanine, and cytosine Parts of nucleic acids • C. Proteins - contain carbon, hydrogen, oxygen, and nitrogen - may also contain sulfur - used to make cell structures, enzymes, hormones, and hemoglobin ▫ 1. chemical structure - made up of amino acids - has an amino group, carboxyl group, central carbon, hydrogen and an R group ▫ 2. polypeptide - chains of amino acids formed by dehydration synthesis – forms peptide bonds Structure of an amino acid: Amino Acids: Amino Acids: Dehydration Synthesis of a dipeptide Peptide bond D. Levels of Structure in Proteins • 1. The primary structure of a protein is its unique sequence of amino acids. • 2. The secondary structure of a protein results from hydrogen bonds at regular intervals along the polypeptide backbone. ▫ Typical shapes that develop from secondary structure are coils (an alpha helix) or folds (beta pleated sheets). Levels of Structure in Proteins • 3. Tertiary structure is determined by a variety of interactions among R groups and between R groups and the polypeptide backbone. ▫ These interactions include hydrogen bonds among polar and/or charged areas and ionic bonds between charged R groups Levels of Structure in Proteins • 4. Quarternary structure results from the aggregation of two or more polypeptide subunits. IV. Enzymes – a type of protein A. importance of enzymes - control chemical reactions - can be used over and over again because they are not changed by the reaction - catalysts • B. structure and function of enzymes ▫ 1. Chemical nature - all enzymes are proteins - may need a coenzyme to help them (usually a vitamin) ▫ 2. Active site - the small region on the enzyme that is involved in the chemical action ▫ 3. Enzyme-substrate complex substrates are the materials that are acted on by the enzyme - when the enzyme and substrate combine they form an enzyme-substrate complex ▫ 4. Lock-and-key model - the shape of the active site fits only one specific substrate ▫ 5. induced fit model – the shape of the active site changes slightly to fit the shape of the substrate ▫ 6. Replacement of enzymes - even though enzymes can be used again they will eventually wear out and new enzymes must be made ▫ 7. Names of enzymes - end in ase and are often named after the substrate they work on - example: maltase breaks down maltose • C. Factors affecting enzyme action ▫ 1. Temperature - enzyme action is generally low at low temp. - as temp. rises so does rate of action however at some point the enzyme becomes denatured and no longer works Enzymes and temperature ▫ 2. Enzyme and substrate concentration - will increase to a point then level off ▫ 3. pH - different enzymes work best at different pH levels Enzymes and pH V. Water Water – An important inorganic compound ▫ 1. cohesion – property of water that allows the water molecules to “stick” to each other ▫ 2. adhesion – property of water that allows the water molecules to “stick” to other substances ▫ 3. capillary action – property of water that allows water to travel upwards against gravity through tiny spaces VI. Functional Groups • The components of organic molecules that are most commonly involved in chemical reactions are known as functional groups. ▫ Functional groups are attachments that replace one or more hydrogen atoms to the carbon skeleton of the hydrocarbon. • A. In a hydroxyl group (-OH), a hydrogen atom forms a polar covalent bond with an oxygen which forms a polar covalent bond to the carbon skeleton. ▫ Because of these polar covalent bonds hydroxyl groups improve the solubility of organic molecules. ▫ Organic compounds with hydroxyl groups are alcohols and their names typically end in –ol --- also found in carbohydrates Copyright © 2002 Pearson Education, Inc., publishing as Benjamin Cummings • B. A carbonyl group (=CO) consists of an oxygen atom joined to the carbon skeleton by a double bond. ▫ If the carbonyl group is on the end of the skeleton, the compound is an aldelhyde. ▫ If not, then the compound is a ketone. Copyright © 2002 Pearson Education, Inc., publishing as Benjamin Cummings • C. A carboxyl group (-COOH) consists of a carbon atom with a double bond with an oxygen atom and a single bond to a hydroxyl group. ▫ Compounds with carboxyl groups are carboxylic acids. ▫ A carboxyl group acts as an acid because the combined electronegativities of the two adjacent oxygen atoms increase the dissociation of hydrogen as an ion (H+). Copyright © 2002 Pearson Education, Inc., publishing as Benjamin Cummings • D. An amino group (-NH2) consists of a nitrogen atom attached to two hydrogen atoms and the carbon skeleton. ▫ Organic compounds with amino groups are amines. ▫ Amino acids, the building blocks of proteins, have amino and carboxyl groups. Copyright © 2002 Pearson Education, Inc., publishing as Benjamin Cummings • E. A sulfhydryl group (-SH) consists of a sulfur atom bonded to a hydrogen atom and to the backbone. ▫ Organic molecules with sulfhydryl groups are thiols. ▫ Sulfhydryl groups help stabilize the structure of proteins. Copyright © 2002 Pearson Education, Inc., publishing as Benjamin Cummings • F. A phosphate group (-OPO32-) consists of phosphorus bound to four oxygen atoms (three with single bonds and one with a double bond). Copyright © 2002 Pearson Education, Inc., publishing as Benjamin Cummings