* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Neurons of the hippocampus form and function
Multielectrode array wikipedia , lookup
Axon guidance wikipedia , lookup
Synaptogenesis wikipedia , lookup
Optogenetics wikipedia , lookup
Neuroanatomy wikipedia , lookup
Development of the nervous system wikipedia , lookup
Anatomy of the cerebellum wikipedia , lookup
Subventricular zone wikipedia , lookup
Feature detection (nervous system) wikipedia , lookup
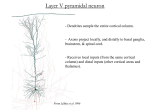
Neurons of the hippocampus form and function Cristian Guatta, Michelle Bachmann Supervised by Stewart Berry in the Lab of Urs Gerber University of Zurich and ETH Zurich, Brain research institute Abstract In this project we examined the unique properties of neurons within the hippocampus, a region of the brain required for learning and memory. Electrical properties of pyramidal cells (PCs) in CA3 and CA1, as well as granule cells (GCs) of the dentate gyrus (DG) were examined via whole-cell path clamp recordings. Their morphology was studied via confocal microscopy.Recoridings were done on organotypic slice cultures that had been grown for three weeks. Introduction1 The Hippocampus is the part of the brain crucial for learning, memory and its retrieving. There are three different areas segmented in dentate gyrus granule cells (GCs) and pyramidal cells (PCs) of CA3 and CA1. Granule cells Granule cells are located in the dentate gyrus (D.G.) and receive inputs from the entorhinal cortex (E.C.)layer II. Dendrites from granule cells project in one direction to the molecular layer. The axons project to CA3 pyramidal cells. CA3 In CA3 inputs come from the E.C. layer II and the D.G. The neurons have basal and apical dendrites. The axons projects to CA1. CA1 CA1 receives inputs from E.C. layer III and CA3. The neurons have basal and apical dendrites. Their Axons project to the subiculum and the E.C. 1 Pictures on page 3 1 Method and Material We performed electrophysiology on organotypic slice cultures prepared from 6 days old mice. The slices had been stored in a mediums for three weeks. Neurons were patched in whole-cell configuration and voltage clamped at -70mV. Spontaneous inward and outward currents were detected and later analyzed using Clampfit software. Action potential, shape and firing patterns were used to confirm the cell types. During the patch experiments a biocytin, which was in the intracellular solution, passively diffused throughout the cell. The neuron could then be treated with streptavidin-Alexa 546, which bound tightly to biocytin. After the patching, the slices were incubated in 4% PFA (paraformaldehyde) for 15 minutes at room temperature. Slices were then washed 3x 10min in 10% PBS (phosphate-buffered saline) at room temperature. Slices were incubated overnight in a blocking solution containing 0.1M PO4, 0.5% triton-x 100 and 10% horse serum, rocking at 4°C. On the next day the SA-Alexa 546 was diluted 1:1000 in 0.1M PO4, 0.5% triton-x 100 and 5% horse serum. A use of 3ml for each slice was approximate. Slices were then incubated overnight, rocking at 4°C. The SA-Alexa was then decanted and the slices were washed 3x 10 minutes in 10% PBS at room temperature. Slices were adhered to glass slides with Dako mount solution. Confocal microscopy was then used. The imaged cell was reconstruct using the software ImageJ. A sholl analysis was then performed. Results We compared the real-time activity of living cells in the three different regions (GC, CA3, CA1) of the hippocampus by using electrophysiology. The frequency of excitatory activity varies among granule and pyramidal cells. Granule cells tend to be more excitable. Confocal microscopy was then used to study the dendritic morphology of these cells and the sites where communication between neurons occur. This was possible through the injected biocytin and the applied SA-Alexa 546, which starts to fluoresced when exposed to light with a wavelength of 546nm (Green light). This allowed us to see the cell body, axon and dendrites. Sholl analyses revealed that there is more dendritic branching in pyramidal cells than there is in granule cells. A token to distinguish between dendrites and axons is their thickness and their morphology. Dendrites are thicker then axons and covered in spines. The axons of the granule cells project in the opposite direction than the dendrites and thus are clearly visible. The axons of the pyramidal cells project in the same direction as their basal dendrites and thus less noticeable. 2 1) Sholl analysis of granule cells a) b) c) A look at the different cells using cofocal microscopy a) Granule cell b) CA3 (only the apical dendrites are shown since we didn’t work with the basal dendrites) c) CA1 3 18 16 14 12 10 8 6 4 2 0 1) Sholl analysis of granule cells 5 20 35 50 65 80 95 110 125 140 155 170 185 200 215 230 245 260 Number of Intersection 1) The software creates circles around the cell body with a distance of 5 um and measures the amount of dendrites in each circle. Distance from Soma 2) 18 16 14 12 10 8 6 4 2 0 345 325 305 285 265 245 225 205 185 165 145 125 105 85 65 45 25 2) Sholl analysis of CA3 pyramidal cells 5 Number of Intersections Sholl Analysis: Distance from Soma Discussion We have shown the differences in excitatory activity among granule cells and pyramidal cells by using electrophysiology. Furthermore, observed differences in morphology of the cell types in area CA3, CA1 and granule cells via confocal microscopy sholl analyses. Our experiments have shown that there is a higher dendritic branching in pyramidal cells than there is in granule cells. This is in agreement with previous observartions. References Greg Miller, How are memories retrieved? (2012); http://goo.gl/QWCyLa Acknowledgment We would like to thank the organization Swiss youth science for organizing this week. Further the university of Zurich and the ETH Zurich for making this week possible. A special thank to Urs Gerber for letting us work in his laboratory and our supervisor Stewart Berry for giving us an insight into electrophysiology and guiding us through the week. We had a lot of fun diving into this field. Thanks everyone who made this week an amazing experience. 4