* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Differential Expression of the Melatonin Receptor in Human
Molecular mimicry wikipedia , lookup
5-Hydroxyeicosatetraenoic acid wikipedia , lookup
Psychoneuroimmunology wikipedia , lookup
Polyclonal B cell response wikipedia , lookup
Cancer immunotherapy wikipedia , lookup
Innate immune system wikipedia , lookup
12-Hydroxyeicosatetraenoic acid wikipedia , lookup
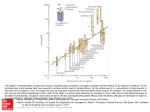
Differential Expression of the Melatonin Receptor in Human Monocytes This information is current as of June 16, 2017. Permissions Email Alerts J Immunol 1998; 160:1191-1197; ; http://www.jimmunol.org/content/160/3/1191 Information about subscribing to The Journal of Immunology is online at: http://jimmunol.org/subscription Submit copyright permission requests at: http://www.aai.org/About/Publications/JI/copyright.html Receive free email-alerts when new articles cite this article. Sign up at: http://jimmunol.org/alerts The Journal of Immunology is published twice each month by The American Association of Immunologists, Inc., 1451 Rockville Pike, Suite 650, Rockville, MD 20852 Copyright © 1998 by The American Association of Immunologists All rights reserved. Print ISSN: 0022-1767 Online ISSN: 1550-6606. Downloaded from http://www.jimmunol.org/ by guest on June 16, 2017 Subscription Marc J. Barjavel, Zahra Mamdouh, Nadjibe Raghbate and Ouahid Bakouche Differential Expression of the Melatonin Receptor in Human Monocytes1 Marc J. Barjavel, Zahra Mamdouh, Nadjibe Raghbate, and Ouahid Bakouche2 T he immune system interacts through its mediators with the brain (1– 4). In return, the neuroendocrine system modulates the immune system through its hormones (5–7). Among the neuroendocrine factors that affect the immune system, the pineal hormone melatonin (MLT)3 appears to be an important one (8, 9). Indeed, melatonin has been shown to enhance the natural and acquired immunity in vivo (10), to activate bone marrow and lymph node cells (11), to activate NK cell activity (9, 12) and Ab-dependent cellular cytotoxicity (13), to enhance the Ab responses in vivo (3, 10, 14), to restore the impaired Th cell activity in immunodepressed mice (14), to increase T cell proliferation in vivo and in vitro (14, 15), and to activate monocytes (8, 16) and neutrophils (17). MLT has also been shown to induce the release of lymphokines (3, 18, 19) such as IL-1 (8, 14), TNF (14), GM-CSF (20), IL-4 (21), IL-2, and IL-5 in vivo (18). In return, IFN-g, thymic hormones, GCSF, GM-CSF, and TNF-a influence the secretion of MLT (3, 4, 9, 16, 22, 23). MLT enhances Ag presentation by splenic macrophages to T cells concomitant with an increase in the expression of MHC class II molecules (14). In addition MLT has immunoenhancing properties. Indeed, MLT in vivo increases the number of Th2 lymphocytes (18, 21), and a com- INSERM U 294, Laboratoire d’Immunologie-Hematologie, CHU Xavier Bichat, Paris, France Received for publication June 12, 1997. Accepted for publication October 22, 1997. The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked advertisement in accordance with 18 U.S.C. Section 1734 solely to indicate this fact. 1 This work was supported by an INSERM grant, a grant from the Association pour la Recherche contre le Cancer, and a grant from the Fondation pour la Recherche Médicale. 2 Address correspondence and reprint requests to Dr. Ouahid Bakouche, INSERM U 294, Laboratoire d’Immunologie-Hematologie, CHU Xavier Bichat, 46 rue Henri Huchard, 75018 Paris, France. 3 Abbreviations used in this paper: MLT, melatonin; GM-CSF, granulocyte-macrophage CSF; MTT, 3-[4,5-dimethylthiazol-2-yl]2,5-diphenyltetrazolium bromide; LBP, LPS-binding protein. Copyright © 1998 by The American Association of Immunologists bination of MLT and IL-2, compared with IL-2 alone, increases the number of T lymphocytes, NK cells, and eosinophils (18, 24 –26). In addition to its immunoregulatory properties, MLT displays oncostatic properties (27–30). For instance, MLT induces the antitumoral functions of human monocytes in vitro (8), inhibits the growth of tumors in vivo (29), and has been used in association with lymphokines for cancer immunotherapy in humans (16, 18, 24 –26, 31). Besides the fact that MLT is a free radical scavenger and can act without the presence of a receptor, the biologic activity of MLT appears also to correlate with the expression of its receptor in target cells. MLT receptors have been described in immune tissues of various nonhuman species (32, 33) and in human immune cells such as T cells (Kd 5 240 pM) (15), human Th2 lymphocytes from bone marrow (Kd 5 346 pM) (21), human platelets (Kd 5 4 nM) (34), human neutrophils (Kd 5 132 pM) (35), and human granulocytes (Kd 5 2 nM) (35). High and low melatonin binding sites have been described (35–37). Also MLT receptors have been detected at plasma membrane levels, in the cytosol, and at the membranes of the nucleus and the mitochondria (38, 39). We report here that human monocytes can be activated by melatonin to induce the release of IL-1, TNF, and IL-6. The purpose of this work was to demonstrate the presence and the expression of MLT receptors on human monocyte cell surface. MLT receptors were found on human monocytes with a Kd around 230 6 60 pM (40,000 receptors/monocyte), and it seems that MLT receptor expression is sensitive to the differentiation of monocytes in vitro. Materials and Methods Chemicals and biologicals LPS (a toxin derived from Escherichia coli) was purchased from Sigma Chemical Co. (St. Louis, MO). Eagle’s MEM and sera were purchased from M. A. Bioproducts (Walkersville, MD). RPMI 1640 was purchased from Mediatech (Herndon, VA). The above medium components were endotoxin free as determined by the Limulus amebocyte lysate assay. Cold melatonin (N-acetyl-5-methoxytryptamine) was purchased from Sigma Chemical Co. (St. Louis, MO). 2-[125I]iodomelatonin (3700 kBq; 100 mCi) and [3H]thymidine were purchased from DuPont-New England Nuclear 0022-1767/98/$02.00 Downloaded from http://www.jimmunol.org/ by guest on June 16, 2017 Earlier studies have shown that the pineal hormone melatonin activates human monocytes. It is reported here that melatonin induces the secretion of IL-1, IL-6, and TNF in fresh and 1-day in vitro cultured monocytes that also express the melatonin receptor (Kd 5 270 6 60 pM; 42,000 – 48,000 receptors/cell). However, when monocytes were cultured in vitro for 2 days, the number of receptors decreased to 11,000 receptors/cell, with the same Kd. LPS activation of fresh or 1-day cultured monocytes did not result in any increase in melatonin receptor number. LPS activation of 2-day cultured monocytes led to an increase in the number of melatonin receptors, from 11,000 receptors/cell to the plateau of 42,000 to 48,000 receptors/cell. The loss of receptors by 2-day cultured monocytes was not irreversible. Melatonin did not induce the release of IL-1, TNF, or IL-6 in monocytes cultured in vitro for 3 days and for up to 15 days, and these long time cultured monocytes did not express the melatonin receptors even after activation by LPS. The loss of melatonin receptors by monocytes cultured in vitro for 3 days and for up to 15 days was irreversible. Therefore, it is shown for the first time that human monocytes express melatonin receptors. Furthermore, human monocytes express melatonin receptors differentially depending on their state of maturation, and it appears that in vitro monocyte differentiation and maturation negatively affect human monocyte melatonin receptor expression. The Journal of Immunology, 1998, 160: 1191–1197. 1192 HUMAN MONOCYTE MELATONIN RECEPTOR EXPRESSION (Boston, MA). The L929 murine fibroblast cell line, the 7TD1 murine hybridoma cell line, and the D10G4.1 T cell clone were purchased from American Type Culture Collection (Rockville, MD). IL-1, IL-6, and TNF were obtained from Genzyme Co. (Boston, MA). Purification of human monocytes Human peripheral blood monocytes were isolated from normal buffy coats using the slightly hypertonic solution and monocyte separation medium Nycoprep 1.068 (Life Technologies, Grand Island, NY) as previously described (8). Monocytes were purified endotoxin free and were found to be 94 to 96% pure as determined by nonspecific esterase staining of fixed cells (8, 40, 41). Activation of monocytes in vitro Monocytes were incubated for 1 h at 37°C, and the nonadherent cells were removed. The immunomodulator (LPS or melatonin) was then added to each culture in triplicate (MEM, without serum), and the cells were incubated for 16 h at 37°C as previously described (8, 40, 41). The supernatants (extracellular TNF, IL-1, and IL-6) were collected, clarified by centrifugation, and assayed for their TNF, IL-1, and IL-6 activities. TNF activity was measured using the standard L929 fibroblast cell line. TNF cytotoxicity toward L929 cells was evaluated using the MTT colorimetric assay as previously described (40, 41). Biologic assay for IL-6 IL-6 activity was measured as previously described (42– 44) using the 7TD1 mouse IL-6-dependent hybridoma proliferation assay. This hybridoma responds mitogenically to IL-6. The proliferation was evaluated using the MTT colorimetric assay. The 7TD1 can accurately measure 50 pg/ml of IL-6. Units of IL-6 activity. Samples were assayed in serial dilutions, and their activities (units) were determined as the reciprocal of the dilutions producing 50% maximal proliferation as described. Biologic assay for IL-1 IL-1 activity was performed using the IL-1-dependent Th cell clone D10G4.1 (D10). D10 cells were incubated on test plates in the presence of 10-ml samples volume for 48 h at 37°C. The D10 proliferation was determined using the MTT assay (40, 41). Samples were assayed in serial dilutions, and their activities (units) were determined as the reciprocal of the dilution producing 50% maximal proliferation (40, 41). 125 [ I]melatonin binding assay Human monocytes or U937 or U373 cells (5 3 105 cells) were heavily washed and incubated in HBSS buffer (Life Technologies; 1 mg/ml aprotinin and 1 mM EDTA, Ca21, MgCl2, MgSO4, and phenol red free) in the presence of 25 to 800 pM 2-[125I]iodomelatonin concentrations (Dupont, Wilmington, DE; 2200 Ci/mmol; 235 mCi/ml) (40). For nonspecific binding, a concentration of 0.8 mM cold melatonin was added. Incubations were performed in 24-well polystyrene plates (Corning, Corning, NY) in a total volume of 500 ml. After 1-h incubation at 37°C, plates were centrifuged (15 min at 300 rpm), and the supernatants were removed with a Pasteur pipette. Pellet cells were washed twice in 500 ml of HBSS buffer to remove unbound melatonin. Cell were solubilized with 500 ml of NaOH (1 mM) and 500 ml of distilled water and collected in borosilicate glass tubes (Baxter). The total 1-ml volume mixture was counted in a gamma counter (model B5002, Packard, Downers Grove, IL). Data were reported as specific binding activity, i.e., total tracer bound minus nonspecific binding. Binding data analysis Before nonlinear regression analysis of binding data were available on computer, binding data were transformed to Scatchard plots, and the binding capacity and Kd were determined using linear regression of the Scatchard plot. Linear regression assumes that the variability assay replicate y values follow a Gaussian distribution, with a SD that does not depend on the value of x. This assumption is rarely true with the transformed data. Linear regression also assumes that x and y are measured independently. This also is not always true. Therefore, it was decided here to present the data as a hyperbola plot to measure binding capacity and Kd, and for general understanding to display FIGURE 1. Scatchard plot analysis of [125I]melatonin binding to human monocytes. Fresh circulating human monocytes isolated from buffy coat (5 3 105 cells) were incubated with [125I]melatonin (0.5–700 pM). Binding assays were performed as described in Materials and Methods. MichaelisMentsen curve (A) and Scatchard plot (B) analysis of [125I]melatonin binding were realized. the most important Scatchard plot, with the best and most accurate representation of the binding data remaining the hyperbola plot. Statistical analysis The statistical significance of difference between test groups was analyzed by Student’s t test (two tailed); p , 0.05 was regarded as statistically significant (40, 41). Results Melatonin receptor expression on fresh monocytes (Fig. 1) To determine whether circulating monocytes express melatonin receptor, binding experiments and Scatchard plot analysis were performed using fresh monocytes and different concentrations of [125I]melatonin. As shown in Figure 1, specific [125I]melatonin binding was saturable in human monocytes and reproducible. Kd ranges were obtained. The Kd for human monocytes was 270 6 60 pM, and human monocytes expressed an average of 41,400 6 4,500 receptors/cell. These data suggest that fresh circulating nonactivated human monocytes do express the receptor for melatonin. Monocyte activation by melatonin (Fig. 2) To determine whether melatonin does activate human monocytes, human monocytes cultured for 1 day were incubated with different concentrations of melatonin (Fig. 2, left). The monocyte activation with MLT was determined as the ability of these MLT-activated monocytes to secrete IL-1, IL-6, or TNF. As shown in Figure 2, Downloaded from http://www.jimmunol.org/ by guest on June 16, 2017 Biologic assay for TNF The Journal of Immunology 1193 melatonin, above a monocyte activation threshold concentration of approximately 50 pM, was able to induce IL-1, IL-6, and TNF. A concentration of 100 pM melatonin allowed a plateau of IL-1, IL-6, and TNF secretion by human monocytes. To determine whether melatonin does activate in vitro cultured and mature monocytes, human monocytes maintained in culture in vitro for several days were then activated with different concentrations of melatonin. As shown in Figure 2 (right), melatonin did not induce significant secretion of IL-6, IL-1, and TNF in monocytes cultured for 3 days in vitro. Indeed, the 3-day cultured monocyte activation threshold concentration was around 80 pM for each cytokine, and the plateau of cytokine secretion was reached for concentrations of melatonin above 100 pM. However, the plateau of cytokine secretion for 3-day cultured and melatonin-activated monocytes represents only 4% of the plateau of cytokine secretion observed for monocytes cultured for 1 day and activated by melatonin. Furthermore, this 4% cytokine secretion plateau is not statistically different from the values obtained for nonactivated monocytes (data not shown). These data showed that melatonin does activate monocytes cultured in vitro for 1 day, but fails to activate monocytes cultured in vitro for 3 days. Differential expression of the melatonin receptor (Fig. 3) Figure 2 showed that melatonin did activate monocytes cultured in vitro for 1 day but failed to activate monocytes cultured in vitro for 3 days. To determine whether the lack of activation of 3-day cultured monocytes by melatonin could be associated with a lack of melatonin receptor expression, binding experiments and Scatchard plot analysis were performed using [125I]melatonin on human monocytes cultured in vitro for different lengths of time (Fig. 3). Fresh circulating monocytes and monocytes cultured in vitro for 1 day expressed the same number of receptors per cell, i.e., 42,000 6 4,000 for fresh monocytes and 48,000 6 2,000 for 1-day cultured monocytes. However, monocytes cultured in vitro for 2 days only expressed 11,000 6 2,000 receptors/cell, i.e., a decrease of 75% compared Downloaded from http://www.jimmunol.org/ by guest on June 16, 2017 FIGURE 2. Melatonin can activate 1-day cultured monocytes. Activation of 1-day cultured monocytes by melatonin was determined by measurements of the secretion of IL-1, IL-6, and TNF (left). Melatonin was used in a concentration range of 0.1–10 nM, and the production of cytokines was calculated as total units per culture using a cell line bioassay specific to each cytokine and the MTT colorimetric assay (see Materials and Methods). The same type of experiment (measurements of the IL-1, IL-6, and TNF-a secretion) is shown on the right, but the activation by melatonin was realized with 3-day cultured monocytes. 1194 HUMAN MONOCYTE MELATONIN RECEPTOR EXPRESSION FIGURE 3. Expression of the melatonin receptor during different days of in vitro culture. Time course of the expression of melatonin receptor at different days in culture at 37°C (black histograms). For comparison, two other cells line, U937 and U373, were represented (white histograms). Data were expressed as the total number of receptors per cell 6 SEM (n 5 5). The number of receptors per cell was measured by Scatchard plot analysis using [125I]melatonin. (0 5 fresh monocytes; 1 5 1-day in vitro cultured monocytes; 2 5 2-day in vitro cultured monocytes; 3 5 3-day in vitro cultured monocytes; 15 5 15-day in vitro cultured monocytes). Effect of LPS monocyte activation on monocyte melatonin receptor expression (Figs. 4-6) Figure 3 shows that monocytes cultured in vitro for 2 days display a loss of 75% in melatonin receptor expression compared with melatonin receptor expression of fresh monocytes or monocytes cultured in vitro for 1 day. To determine whether the loss of melatonin receptor expression observed in monocytes cultured for 2 days is reversible, fresh monocytes and monocytes cultured in vitro for either 1 or 2 days were or were not activated with 1 mg/ml of LPS before performing binding experiments using [125I]iodomelatonin. As shown in Figures 4 and 6, LPS activation did not increase the number of melatonin receptors in fresh monocytes or monocytes cultured for 1 day (nonactivated fresh monocytes, 42,000 6 6,000 receptors/cell; LPS-activated fresh monocytes, 43,000 6 8,000 receptors/cell; nonactivated 1-day monocytes, 48,000 6 7,700 receptors/cell; LPS-activated 1-day cultured monocytes: 45,000 6 8,000 receptors/cell). However (Figs. 5 and 6), LPS activation increased the number of melatonin receptors per cell in monocytes cultured in vitro for 2 days from 10,800 6 1,880 to 42,800 6 9,000 melatonin receptors/cell. The FIGURE 4. Effect of LPS activation on the expression of melatonin receptor by fresh and 1-day cultured human monocytes. The binding of [125I]melatonin on monocytes and the saturation curve were determined using different [125I]melatonin concentrations on nonactivated monocytes (fresh monocytes (A) and 1-day cultured monocytes (B)) and LPS-activated monocytes (fresh monocytes (C) and 1-day cultured monocytes (D)). Data are expressed as the number of receptors per cell determined for each concentration of [125I]melatonin by Scatchard plot analysis. Downloaded from http://www.jimmunol.org/ by guest on June 16, 2017 with the number of receptors expressed in fresh and 1-day cultured monocytes. After 2 days of culture in vitro, monocytes did not express melatonin receptors. We investigated whether U937 monocytic cells and U373 astrocytoma cells did express melatonin receptors. As shown in Figure 3, U373 cells expressed 12,000 6 2,000 receptors/cell, whereas U937 did not express receptors for melatonin. These data suggest that long term culture of monocytes and/or cellular maturation/differentiation induces a loss of melatonin receptors in monocytes. The Journal of Immunology 1195 number of melatonin receptors in LPS-activated 2-day cultured monocytes reached a level identical with that in nonactivated fresh monocytes (42,000 receptors/cell), LPS-activated fresh monocytes (43,000 receptors/cell), nonactivated 1-day cultured monocytes (48,000 receptors/cell), and LPS-activated 1-day cultured monocytes (45,000 receptors/cell). Therefore, the maximum amount of melatonin receptors, i.e., the plateau of melatonin receptor number per cell is around 42,000 to 48,000 receptors/cell. It is interesting to note that LPS activation increased the number of melatonin receptors in 2-day cultured monocytes, and that the affinity of melatonin toward these receptors remained identical (Kd 5 270 6 60 pM) in all cases, i.e., nonactivated and LPS-activated fresh monocytes and 1- or 2-day cultured monocytes. The LPS activation did not affect the affinity of melatonin for its receptors. Therefore, it FIGURE 6. Melatonin receptor expression on cultured monocytes. Melatonin receptors were expressed as the maximal number of receptors per cell for nonactivated monocytes (black histograms) and for LPSactivated monocytes (white histograms). Data were expressed as the mean 6 SEM (n 5 5); p , 0.05 vs activated cells. 0 5 Fresh monocytes; 1 5 1-day in vitro cultured monocytes; 2 5 2-day in vitro cultured monocytes; 3 5 3-day in vitro cultured monocytes; 15 5 15-day in vitro cultured monocytes. appears that LPS does increase the number of melatonin receptors in 2-day cultured monocytes. Effect of LPS activation on monocytes lacking melatonin receptor expression (Fig. 6) Our previous results showed that LPS activation can restore the level of melatonin receptor expression in monocytes cultured in vitro for 2 days to the level observed in fresh monocytes or in monocytes cultured in vitro for 1 day. Also, LPS activation had no effect on the expression of melatonin receptors by fresh or 1-day cultured monocytes (Figs. 4 and 6). Figure 3 showed that monocytes cultured in vitro for .2 days did not express melatonin receptors. To determine whether monocytes cultured for .2 days Downloaded from http://www.jimmunol.org/ by guest on June 16, 2017 FIGURE 5. Effect of LPS activation on melatonin receptor expression by 2-day cultured human monocytes. Saturation curves and binding of [125I]melatonin on monocytes were determined using different [125I]melatonin concentrations on nonactivated 2-day cultured monocytes (left) and on 2-day LPS-activated monocytes (right). Data are expressed as the total number of receptors complexed with [125I]melatonin for the different melatonin concentrations used. 1196 and activated by LPS could express melatonin receptors, monocytes cultured in vitro for 3 and 15 days were activated by 1 mg/ml of LPS before performing binding experiments and Scatchard plot analysis using [125I]melatonin. Figure 6 shows that LPS activation failed to induce the expression of melatonin receptors in 3- or 15-day cultured monocytes. Therefore, the loss of melatonin receptor expression in monocytes cultured for .2 days in vitro cannot be reversed by LPS activation. Discussion sites with an affinity constant that is the average between that of high affinity sites (Kd 5 ;1–30 pM) and that of low affinity sites (Kd 5 ;1–5 nM). MLT binding sites have also been described at the plasma membranes (32), in the cytosol (39), at the mitochondria membranes (38), and at the nucleus membranes (38). Furthermore, Steinhilber et al. have described a nuclear receptor for MLT, shown that MLT is a natural ligand of RZR a and b, and proposed that ligand-induced transcriptional control could mediate physiologic functions (51). Because we used whole monocytes in our studies, it is difficult to determine where the MLT binding sites are in human monocytes. Our results also show that the expression of MLT receptors in human monocytes is correlated with the state of in vitro maturation of the monocytes. Indeed, fresh monocytes (monocytes just purified from the blood donor) and monocytes in culture for 1 day expressed the maximum number of MLT receptors (plateau at 40,000 receptors/cell). After 2 days in culture, the number of MLT receptors dropped to 11,000 receptors/cell. After 3 days in culture, the monocytes did not express MLT binding sites. In addition, it has been shown that the maturation process of monocytes in vitro is completed after 3 days in culture, and the circulatory monocytes become differentiated macrophages (52). It seems from our results that it takes 3 days of in vitro culture for monocytes to lose the expression of MLT receptors, and it takes 3 days of in vitro culture for monocytes to differentiate into macrophages. It has been reported that MLT regulates its own receptor and maintains the presence of its receptor on cells (53). Our hypothesis is that as long as monocytes remain in contact with MLT they express the MLT receptor. However, when monocytes are kept away from MLT for a sufficient time and when they mature, they lose the expression of the MLT receptors. Therefore, circulating monocytes and freshly isolated monocytes do express MLT receptors, probably because they have been in contact with MLT in the recent past and also because they are not yet differentiated. After a certain time without MLT (culture in vitro for 2 days), the number of MLT receptors declines. This is an intermediary state between circulating monocytes and fully maturated monocytes in vitro. Finally, after 3 days of in vitro culture, monocytes have been for a sufficient time without MLT and are differentiated into mature monocytes in vitro. They do not express MLT receptors. This loss of MLT receptor expression in monocytes cultured in vitro for 3 days or more appears to be irreversible, since activation by LPS did not restore the expression of MLT receptors by monocytes. However, the decline in MLT receptor expression observed in monocytes cultured in vitro for 2 days appears to be reversible, since LPS activation of these monocytes restored the number of MLT receptors per cell to its original plateau value observed for freshly isolated monocytes (40,000 receptors/cell). The decline in MLT receptor expression observed for monocytes cultured in vitro for 2 days is reversible (transitory in vitro monocyte maturation stage), whereas the loss of MLT receptor expression observed for monocytes cultured in vitro for 3 days is irreversible. As a consequence, MLT can still induce the release of IL-1, TNF, and IL-6 in monocytes cultured in vitro for 2 days but fail to induce the secretion of these three cytokines in monocytes cultured in vitro for 3 days. Finally, it should be noted that although the number of MLT receptors declines when monocytes are cultured for a long time in vitro, the affinity of these MLT receptors for MLT remains the same. In closing, it seems that the monocyte expression of the MLT receptors closely depends on the state of maturation and differentiation of the monocytes and that MLT must maintain the expression of its own receptor on monocytes. Downloaded from http://www.jimmunol.org/ by guest on June 16, 2017 For the first time it is reported here that human monocytes express melatonin receptors, and the melatonin receptor expression is dependent on the monocyte state of maturation. Indeed, we report here that MLT induces the release of IL-1, TNF, and IL-6 in human monocytes and that human monocytes express a single class of receptors with a Kd of approximately 270 6 80 pM. There are about 41,500 6 4,000 MLT receptors/ monocyte. The binding of MLT to the monocyte receptors was saturable, specific, and of high affinity. The presence of the MLT receptor on human monocytes varies with the state of differentiation of the monocytes in vitro. The major cell surface receptor for LPS on monocytes/macrophages is CD14, and the action of LPS is mediated and enhanced by LPS binding protein (LBP) (45). The numbers of CD14 on the cell surface of fresh, 1-, 2-, 3-, and 15-day cultured monocytes were the same (data not shown). Moreover, the system CD14/LBP is involved for LPS concentrations ,10 ng/ml or equal to 10 ng/ml (45– 47); above this concentration of 10 ng/ml, LPS can activate monocyte/macrophage using a pathway independent of CD14/LBP (46, 47). In our studies we used an LPS concentration of 1 mg/ml well above 10 ng/ml; therefore, the system CD14/LBP was not involved. In addition, it has recently been shown that LPS, even at concentrations ,10 ng/ml, can activate monocytes/macrophages through a pathway independent of CD14/ LBP and the tyrosine kinases (46, 47). Therefore, the lack of melatonin receptor expression recovery by LPS in 3-day in vitro cultured monocytes was not due to deficient CD14 expression or signal transduction. MLT has been shown to modulate the secretion of lymphokines (3, 8, 14, 18 –21). MLT induces in vivo the secretion of IL-4 (21), IL-5 (18), GM-CSF (20), and IL-2 (18). Interestingly, in vitro, MLT inhibits the secretion of IL-2 and IFN-g by T cells (48, 49). This discrepancy observed for IL-2 secretion in vivo and in vitro is difficult to explain. MLT also induces the secretion of IL-1 in vitro (8) and in vivo (14), and induces the secretion of TNF-a in vivo (14). Our results show for the first time that MLT induces the secretion of IL-6 and TNF-a by human monocytes in vitro. These results are in agreement with the general idea that MLT induces the secretion of lymphokines by immune cells (3, 8, 14, 18 –21, 49). It seems that the action of MLT on target cells is correlated with the expression of MLT receptors by these target cells. Indeed, MLT receptors have been described in many species (33–35) and on human immune cells such as T lymphocytes (15), bone narrow cells and Th2 lymphocytes (21), platelets (34), neutrophils (35), and granulocytes (35). We report that human monocytes do express a single class of binding sites with a Kd of 270 6 60 pM. These binding sites are specific for MLT, can be saturated, and have the characteristics of a MLT receptor (Scatchard plot analysis). The Kd observed for the monocyte MLT receptors appeared to correlate with that observed for most human immune cells. MLT receptors of high and low affinity have been described; subtypes of high affinity MLT receptors have also been reported (35, 37, 50). It seems that in human monocytes, MLT binds to a single class of HUMAN MONOCYTE MELATONIN RECEPTOR EXPRESSION The Journal of Immunology References 27. Buswell, R. S. 1975. Letter: the pineal and neoplasia. Lancet 1:34. 28. Das Gupta, T. K., and J. Terz. 1967. Influence of pineal gland on the growth and spread of melanoma in the hamster. Cancer Res. 27:1306. 29. Aubert, C., P. Janiaud, and J. Lecalvez. 1980. Effect of pinealectomy and melatonin on mammary tumor growth in Sprague-Dawley rats under different conditions of lighting. J. Neural Transm. 47:121. 30. Lissoni, P., S. Barni, S. Crispino, G. Tancini, and F. Fraschini. 1989. Endocrine and immune effects of melatonin therapy in metastatic cancer patients. Eur. J. Cancer Clin. Oncol. 25:789. 31. Lissoni, P., S. Barni, G. Tancini, V. Fossati, and F. Frigerio. 1994. Pineal-opioid system interactions in the control of immunoinflammatory responses. Ann. NY Acad. Sci. 741:191. 32. Pang, C. S., and S. F. Pang. 1992. High affinity specific binding sites of 2-[125I]iodomelatonin by spleen membrane preparations of chicken. J. Pineal Res. 12:167. 33. Liu, Z. M., and S. F. Pang. 1992. Binding of [125I]-labelled iodomelatonin in the duck thymus. Biol. Signals 1:250. 34. Vacas, M. I., M. M. Del Zar, M. Martinuzzo, and D. P. Cardinali. 1992. Binding sites for [3H]-melatonin in human platelets. J. Pineal Res. 13:60. 35. Lopez-Gonzalez, M. A., J. R. Calvo, J. J. Segura, and J. M. Guerrero. 1993. Characterization of melatonin binding sites in human peripheral blood neutrophils. Biotechnol. Ther. 4:253. 36. Lopez-Gonzalez, M. A., J. R. Calvo, C. Osuna, and J. M. Guerrero. 1992. Interaction of melatonin with human lymphocytes: evidence for binding sites coupled to potentiation of cyclic AMP stimulated by vasoactive intestinal peptide and activation of cyclic GMP. J. Pineal Res. 12:97. 37. Lopez-Gonzalez, M. A., J. R. Calvo, C. Osuna, A. Rubio, and J. M. Guerrero. 1992. Synergistic action of melatonin and vasoactive intestinal peptide in stimulating cyclic AMP production in human lymphocytes. J. Pineal Res. 12:174. 38. Persengiev, S. P. 1992. 2-(125I)iodomelatonin binding sites in rat adrenals: pharmacological characteristics and subcellular distribution. Life Sci. 51:647. 39. Cohen, M., D. Roselle, B. Chabner, T. J. Schmidt, and M. Lippman. 1978. Evidence for a cytoplasmic melatonin receptor. Nature 274:894. 40. McLachlan, J. A., C. D. Serkin, and O. Bakouche. 1996. Dehydroepiandrosterone modulation of lipopolysaccharide-stimulated monocyte cytotoxicity. J. Immunol. 156:328. 41. McLachlan, J. A., C. D. Serkin, K. M. Morrey, and O. Bakouche. 1995. Antitumoral properties of aged human monocytes. J. Immunol. 154:832. 42. Krakauer, T. 1993. A sensitive, specific immunobioassay for quantitation of human interleukin 6. J. Immunoassay 14:267. 43. Proudfoot, A. E., S. C. Brown, A. R. Bernard, J. Y. Bonnefoy, and E. H. Kawashima. 1993. Recombinant human IL-6 expressed in E. coli undergoes selective N-terminal degradation: evidence that the protein consists of a stable core and a nonessential flexible N-terminal. J. Protein Chem. 12:489. 44. Topley, N., A. Jorres, W. Luttmann, M. M. Petersen, M. J. Lang, K. H. Thierauch, C. Muller, G. A. Coles, M. Davies, and J. D. Williams. 1993. Human peritoneal mesothelial cells synthesize interleukin-6: induction by IL-1 beta and TNF alpha. Kidney Int. 43:226. 45. Wright, S. D., R. A. Ramos, P. S. Tobias, R. J. Ulevitch and J. C. Mathison. 1990. CD14, a receptor for complexes of lipopolysaccharide (LPS) and LPS binding protein. Science 249:1431. 46. Perera, P., S. N. Vogel, G. R. Detore, A. Haziot, and S. M. Goyert. 1997. CD14dependent and CD14 independent signaling pathways in murine macrophages from normal and CD14 knockout mice stimulated with LPS or taxol. J. Immunol. 158:422. 47. Meng, F., and C. A. Lowell. 1997. LPS-induced macrophage activation and signal transduction in the absence of Src-family kinases Hck, Fgr and Lyn. J. Exp. Med. 185:1661. 48. Hellstrand, K., and S. Hermodsson. 1990. Monocyte-mediated suppression of IL-2-induced NK-cell activation: regulation by 5-HT1A-type serotonin receptors. Scand. J. Immunol. 32:183. 49. Finocchiaro, L. M., E. S. Arzt, S. Fernandez-Castelo, M. Criscuolo, S. Finkielman, and V. E. Nahmod. 1988. Serotonin and melatonin synthesis in peripheral blood mononuclear cells: stimulation by interferon-gamma as part of an immunomodulatory pathway. J. Interferon Res. 8:705. 50. Pang, S. F., E. A. Ayre, A. M. Poon, C. S. Pang, H. Yuan, Z. P. Wang, Y. Song, and G. M. Brown. 1993. Effects of guanosine 59-O-(3-thiotriphosphate) on 2-[125I]iodomelatonin binding in the chicken lung, brain and kidney: hypothesis of different subtypes of high affinity melatonin receptors. Biol. Signals 2:27. 51. Steinhilber, D., M. Brungs, O. Werz, I. Wiesenberg, C. Danielsson, J. P. Kahlen, S. Nayeri, M. Schrader, and C. Carlberg. 1995. The nuclear receptor for melatonin represses 5-lipoxygenase gene expression in human B lymphocytes. J. Biol. Chem. 270:7037. 52. Janson, R. W., F. G. Joslin, and W. P. Arend. 1990. The effects of differentiating agents on IL-1 beta production in cultured human monocytes. J. Immunol. 145: 2161. 53. Weaver, D. R., S. A. Rivkees, and S. M. Reppert. 1989. Localization and characterization of melatonin receptors in rodent brain by in vitro autoradiography. J. Neurosci. 9:2581. Downloaded from http://www.jimmunol.org/ by guest on June 16, 2017 1. Blalock, J. E. 1984. Relationships between neuroendocrine hormones and lymphokines. Lymphokines 9:1. 2. Rothwell, N. J. 1991. Functions and mechanisms of interleukin 1 in the brain. Trends Pharmacol. Sci. 12:430. 3. Maestroni, G. J. 1995. T-helper-2 lymphocytes as a peripheral target of melatonin. J. Pineal Res. 18:84. 4. Zylinska, K., J. Komorowski, T. Robak, S. Mucha, and H. Stepien. 1995. Effect of granulocyte-macrophage colony stimulating factor and granulocyte colony stimulating factor on melatonin secretion in rats in vivo and in vitro studies. J. Neuroimmunol. 56:187. 5. Maclean, D., and S. Reichlin. 1981. Neuroendocrinology and the immune process. In Psychoneuroendocrimmunology. R. Ader, ed. Academic Press, New York, p. 475. 6. Hall, D., and A. L. Goldstein. 1981. Neurotransmitters and the immune system. In Psychoneuroendocrimmunology. R. Ader, ed. Academic Press, New York, p. 521. 7. Koff, W. C., and M. A. Dunegan. 1985. Modulation of macrophage-mediated tumoricidal activity by neuropeptides and neurohormones. J. Immunol. 135: 350. 8. Morrey, K. M., J. A. McLachlan, C. D. Serkin, and O. Bakouche. 1994. Activation of human monocytes by the pineal hormone melatonin. J. Immunol. 153: 2671. 9. Guerrero, J. M., and R. J. Reiter. 1992. A brief survey of pineal gland-immune system interrelationships. Endocr. Res. 18:91. 10. Poon, A. M., Z. M. Liu, C. S. Pang, G. M. Brown, and S. F. Pang. 1994. Evidence for a direct action of melatonin on the immune system. Biol. Signals 3:107. 11. Wajs, E., E. Kutoh, and D. Gupta. 1995. Melatonin affects proopiomelanocortin gene expression in the immune organs of the rat. Eur. J. Endocrinol. 133:754. 12. del Gobbo, V., V. Libri, N. Villani, R. Calio, and G. Nistico. 1989. Pinealectomy inhibits interleukin-2 production and natural killer activity in mice. Int. J. Immunopharmacol. 11:567. 13. Giordano, M., and M. S. Palermo. 1991. Melatonin-induced enhancement of antibody-dependent cellular cytotoxicity. J. Pineal Res. 10:117. 14. Pioli, C., M. C. Caroleo, G. Nistico, and G. Doria. 1993. Melatonin increases antigen presentation and amplifies specific and non specific signals for T-cell proliferation. Int. J. Immunopharmacol. 15:463. 15. Konakchieva, R., S. Kyurkchiev, I. Kehayov, P. Taushanova, and L. Kanchev. 1995. Selective effect of methoxyindoles on the lymphocyte proliferation and melatonin binding to activated human lymphoid cells. J. Neuroimmunol. 63: 125. 16. Lissoni, P., S. Pittalis, F. Brivio, E. Tisi, F. Rovelli, A. Ardizzoia, S. Barni, G. Tancini, G. Giudici, A. Biondi, and et al. 1993. In vitro modulatory effects of interleukin-3 on macrophage activation induced by interleukin-2. Cancer 71: 2076. 17. Lopez-Gonzalez, M. A., J. R. Calvo, C. Osuna, A. Rubio, and J. M. Guerrero. 1992. Melatonin potentiates cyclic AMP production stimulated by vasoactive intestinal peptide in human lymphocytes. Neurosci. Lett. 136:150. 18. Lissoni, P., S. Barni, G. Tancini, F. Brivio, E. Tisi, B. Zubelewicz, and R. Braczkowski. 1994. Role of the pineal gland in the control of macrophage functions and its possible implication in cancer: a study of interactions between tumor necrosis factor-alpha and the pineal hormone melatonin. J. Biol. Regul. Homeostatic Agents 8:126. 19. Waldhauser, F., B. Ehrhart, and E. Forster. 1993. Clinical aspects of the melatonin action: impact of development, aging, and puberty, involvement of melatonin in psychiatric disease and importance of neuroimmunoendocrine interactions. Experientia 49:671. 20. Maestroni, G. J. 1993. The immunoneuroendocrine role of melatonin. J. Pineal Res. 14:1. 21. Maestroni, G. J., V. Covacci, and A. Conti. 1994. Hematopoietic rescue via T-cell-dependent, endogenous granulocyte-macrophage colony-stimulating factor induced by the pineal neurohormone melatonin in tumor-bearing mice. Cancer Res. 54:2429. 22. Withyakumnarkul B, K. O. Nonaka, C. Santana, A. M. Attia, and R. J. Reiter. 1990. Interferon g modulates melatonin production in rat pineal glands in organ culture. J. Interferon Res. 10:403. 23. Withyakumnarkul, B., K. O. Nonaka, A. M. Attia, and R. J. Reiter. 1990. Changes in indole metabolism in organ cultured rat pineal gland induced by interferon. J. Pineal Res. 8:313. 24. Lissoni, P., A. Ardizzoia, E. Tisi, F. Rossini, S. Barni, G. Tancini, A. Conti, and G. J. Maestroni. 1993. Amplification of eosinophilia by melatonin during the immunotherapy of cancer with interleukin-2. J. Biol. Regul. Homeostatic Agents 7:34. 25. Lissoni, P., S. Barni, M. Cazzaniga, A. Ardizzoia, F. Rovelli, F. Brivio, and G. Tancini 1994:. Efficacy of the concomitant administration of the pineal hormone melatonin in cancer immunotherapy with low-dose IL-2 in patients with advanced solid tumors who had progressed on IL-2 alone. Oncology 51:344. 26. Barni, S., P. Lissoni, M. Cazzaniga, A. Ardizzoia, F. Paolorossi, F. Brivio, M. Perego, G. Tancini, A. Conti, and G. Maestroni. 1992. Neuroimmunotherapy with subcutaneous low-dose interleukin-2 and the pineal hormone melatonin as a second-line treatment in metastatic colorectal carcinoma. Tumori 78:383. 1197