* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Single TMS Receptors
Endomembrane system wikipedia , lookup
Purinergic signalling wikipedia , lookup
Protein moonlighting wikipedia , lookup
NMDA receptor wikipedia , lookup
Phosphorylation wikipedia , lookup
List of types of proteins wikipedia , lookup
Protein phosphorylation wikipedia , lookup
G protein–coupled receptor wikipedia , lookup
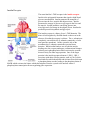
Single TMS Receptors Bryant Miles The single transmembrane segment receptors are proteins with a single transmembrane segment. The transmembrane segment is of course a α−helix. On either side of this membrane spanning helix are globular domains. On the extracellular side is the binding site for the hormone, on the cystolic side of the membrane there is a catalytic domain, typically a tyrosine kinase or guanylate cyclase. For our first example consider the human growth hormone and its receptor. The human growth hormone is a polypeptide hormone of 217 amino acids that forms a compact structure shown below (A). The human growth hormone receptor is a single transmembrane segment receptor. The function of this hormone/receptor signal transduction system is typical for these 1-TMS receptors. In the absence of the human growth hormone, the corresponding receptor exists in a monomeric state. When the growth hormone binds to this receptor, the binding promotes dimerization of two growth receptor molecules. One monomeric receptor binds the red portion of the human growth hormone and the other monomeric receptor binds to the orange portion. The binding of the hormone and the dimerization are very cooperative processes. The dimerization brings together the globular portions (JAK2, Janus kinase 2) of the receptor on the cystolic side of the membrane. The JAK2 domains are tyrosine kinases. Dimerization brings together the 2 JAK2 domains of the receptor. The two tyrosine kinases cross phosphorylate each other which causes conformational changes that activate the two kinases for other protein substrates. After the JAK2 enzymes have been activated by cross phosphorylation, the tyrosine kinase can phosphorylate other substrates. One important substrate is STAT5 (STAT, Signal Transducers and Activators of Transcription). JAK2 phosphorylates a tyrosine residue near the carboxyl terminus of STAT5. This phosphorylated tyrosine binds to a binding site of another STAT5 molecule forming a stable dimer. The dimerized STAT has high affinity for specific binding sites on DNA which regulate gene expression. Other examples are the epidermal growth factor and its receptor and the platlet derived growth factor and its receptor. The receptors of both of these hormones exist in a monomeric state until they bind their corresponding hormone which promotes dimerization. The dimerization induces cross phosphorylation by the receptors tyrosine kinases. The cross phosphorylation activates the tyrosine kinases to phosphorylate other substrate molecules which coordinate cellular processes Small G-proteins We have discussed the heterotrimeric G proteins. Now let’s turn our attention to the small G proteins. These are small proteins that bind guanosine nucleotides and have intrinsic GTPase activity. The small G proteins constitute a huge superfamily of proteins which include Ras, Rho, Arf, Rab and Ran. These small G proteins regulate a large number of cellular processes including growth, differentiation, cell motility, cytokinesis and cellular transport. They are homologous the heterotrimer G proteins and cycle between binding GDP and GTP. The major differences between these two classes of G proteins is that the small G proteins are monomeric and small. The activated form of the small G proteins is the GTP-bound form. We are going to look the small G protein Ras. Ras stimulates cell growth and differentiation. The epidermal growth factor (EGF) and its receptor activate Ras. The binding of EGF causes the receptor to dimerize and cross phosphorylate itself activating the receptor tyrosine kinase. An adaptor protein called Grb-2 binds to the phosphorylated tyrosine residue of the receptor kinase. Grb-2 is one example of large family of proteins that bind to phosphorylated tyrosine residues. The domain that binds the phosphoryated tyrosine residues is called the Src-homology domain 2 or SH2 for short. This adaptor protein then recruits a protein called Sos which interacts with Grb-2. The bound Sos then binds inactive Ras which has GDP tightly bound. The binding of Ras to the Sos-Grb-2-receptor complex causes the guanosine nucleotide binding site to open up releasing GDP and binding GTP. For this reason, Sos is called the guanosine nucleotide exhange factor (GEF). When Ras has GTP bound it is active and dissociates from the complex. Ras like Gα proteins has an intrinsic GTPase activity which functions like a timer terminating the signal, returning Ras to its inactive state. The signaling pathway downstream of Ras activation is shown to the left. A cascade of phosphorylations activate a number of protein kinases which regulate many cellular functions. Ras with GTP bound activates Raf kinase which then activates MEK, MAP kinase kinase, which activates MAP kinase, MAPK. MAPK migrates from the cytosol to the nucleus where it phosphorylates transcription factors that induce the transcription of specific genes. Oncogenes Oncogenes are genes that have the potential to cause a normal cell to become cancerous. Cell growth and differentiation are tightly controlled processes. Tumor cells are uncontrollable rapidly proliferating cells which can grow in invasive manner and threaten life. Cancer is responsible for 20% of all deaths each year. Recently viruses that carry oncogenes that induce tumors have been discovered. Rous Sarcoma Virus (RSV) induces the formation of sarcomas in chickens. The gene responsible for the sarcomas is v-src. The viral gene v-src is homologous to a chicken gene, c-src. Both v-src and c-src encode a tyrosine kinase. The c-src tyrosine kinase is strictly regulated while the v-src is completely unregulated. The v-src tyrosine knase causes uncontrolled cellular proliferation producing the sarcoma. Insulin Receptor The most familiar 1-TMS receptor is the insulin receptor. Insulin is the polypeptide hormone that signals a high blood glucose concentration. Insulin promotes the transport of glucose into the liver, muscle and adipose tissue. Insulin promotes the storage of glucose as glycogen in the liver and the muscle. Insulin promotes converting glucose into triacylglycerols for storage in the adipose tissue. Insulin also promotes protein biosynthesis and glycolysis. The insulin receptor is a dimer of two 1-TMS domains. The dimer is held together by disulfide bonds so that even in the absence of insulin the receptor is a dimer. The α−subunits are completely extracellular, the β−subunits contain the α−helix that spans the membrane. On the cytostolic side of the membrane the β−subunits contain the tyrosine kinase domains. When insulin binds to one or both the insulin binding sites, the receptor undergoes conformational changes inducing cross phosphorylation which activates the tyrosine kinase activity for other target proteins. One of the target proteins is the insulin receptor substrate-1, IRS-1. IRS-1 associates with other effector proteins, such as Grb-2 which then binds Sos which then binds and activates Ras which start a phosphorylation cascade leading to the phosphorylation of MAPK which activates the kinase which then migrates from the cytosol to the nucleus where it phosphorylates transcription factors regulating gene expression.