* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Transamination and asymmetry in glutamate transport across the
Catalytic triad wikipedia , lookup
Basal metabolic rate wikipedia , lookup
Citric acid cycle wikipedia , lookup
Western blot wikipedia , lookup
Genetic code wikipedia , lookup
Human digestive system wikipedia , lookup
Oxidative phosphorylation wikipedia , lookup
Magnesium transporter wikipedia , lookup
Biochemistry wikipedia , lookup
Glyceroneogenesis wikipedia , lookup
Clinical neurochemistry wikipedia , lookup
Amino acid synthesis wikipedia , lookup
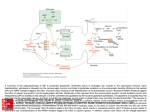
Bioscience Reports i, 851-856 (1981) Printed in Great Britain gSl T r a n s a m i n a t i o n and a s y m m e t r y in g l u t a m a t e t r a n s p o r t across the b a s o l a t e r a l m e m b r a n e of f r o g s m a l l i n t e s t i n e C. A. R. 5OYD and V. S. PERRING Department of Human Anatomy, University of Oxford, South Parks Road, Oxford OXI 3QX, U.K. (Received 20 October 1981) Transport of L - g l u t a m a t e across the b a s o I a t e r a l m e m b r a n e of frog small-intestinal epithelium, unlike that of L-alanine, is highly asymmetric; thus the rate c o n s t a n t (.Kentry) describing the entry of glutamate into the eplthenum from the vascular bed across this membrane is one order of magnitude greater than the r a t e c o n s t a n t ( K e x i t ) d e s c r i b i n g its exit. This asymmetry, which appears not to depend upon the Na g r a d i e n t , may be i m p o r t a n t in maintaining a high i n t r a c e l l u l a r c o n c e n t r a t i o n of glutamate relative to a l a n i n e t h e r e b y favouring the production of alanine from glutamate by transamination. It has long been recognized that the small intestine is a major site of metabolism of e-amino N (Dent & Schilling, 1949; Wiseman, 1953; Windmueller & Spaeth, 1974, 1975; Hanson & Parsons, 1977). Thus for e x a m p l e t r a n s a m i n a t i o n of g l u t a m a t e , c a t a l y s e d by the enzyme giutamic-alanine transaminase (EC 2.6.1.2), results i n the production of alanine through the reaction Ki glutamic acid + pyruvate , 9 alanine + e-ketoglutarate. (1) K2 This reaction is readily reversible ( K I / K 2 = 1.6; Boyer, 1973) and the forward reaction will be favoured by a high cellular concentration of g l u t a m a t e r e l a t i v e to alanine. As the intraepithelial amino acid concentrations will be determined, in part, by their cellular entry and exit mechanisms, we have investigated the transport systems available f o r g l u t a m a t e and a l a n i n e e n t r y and exit across the basolateral membrane of frog (Rana ridibunda) intact small intestine. Methods Evidence that frog small in vitro intestine converts glutamate to alanine Everted rings of R. ridibunda small intestine were incubated for 60 min at 20~ in Frog Ringer solution containing t0 mM L-glutamate, 1 mM D-glucose, and [l#C]inulin (an extracellular marker); as a c o n t r o l , rings were i n c u b a t e d in a s i m i l a r medium from which 9 The Biochemical Society 852 BOYD & PERRING glutamate had been omitted. After incubation, the tissue was removed from the solutions and extracted in 0.04 M HNO 3 overnight at g0~ Samples of the extract were analysed by thin-layer chromatography (Brenner et aJ., 1962). Extracts of the control tissue gave rise to two barely visible Ninhydrin-positive spots (Rf values 0.19 and 0.27); in contrast, extracts of the tissue incubated with glutamate produced seven spots after develpment with Ninhydrin, including two prominent, purple-staining spots with Rf values 0.19 and 0.37, which were shown by comparison with amino acid 'standards' to be glutamate and alanine respectively. Measurement alan• of basolateral membrane transport of glutamate and The vascularly perfused preparation of R. ridibunda small intestine (Boyd et al., 1975) is ideally suited to the study of basolateralmembrane solute transport. Washout of solute from the luminally or vascularly preloaded epithelium into the vascular bed is c h a r a c t e r i z e d by a rate constant, Kexit. This describes exit from the epithelium into the vascular bed across the basolateral membrane. Wash-in to the tissue from the vascular bed (Boyd & Parsons, 1979) enables a f u r t h e r r a t e c o n s t a n t , Ken.try , to be calculated. Rat e constants describing glutamate and alanme entry and exit across the basolateral membrane are presented in Table 1. Results and Discussion Exit from the luminally preloaded epithelium Fig. l shows the vascular appearance of alanine and glutamate during washout from the luminally preloaded epithelium. It is clear that alanine washout is biphasic (as is that of leucine; cf. Cheeseman, 1979), t h e initial phase being c h a r a c t e r i z e d by Kexit (fast, Table I. Rate constants describing the transport of alanine and glutamate across the basolateral face of the vascularly perfused epithelium of R. ridibunda small intestine Values are means • S.E.M.~ with the number of samples shown in parentheses. Kentry (min -I ) for wash-in to epithelium from vascular bed Kexit (min -I) for washout from epithelium into vascular bed following loading from Lumen Alanine Glutamic acid 0.196 • 0.019 (ii) 0.327 • 0.116 (i0) Vascular bed 0.150 • 0.008 (14) fast (unstripped) 0.181 • 0.051 (8) fast (unstripped) 0.044 • 0.004 (14) slow 0.031 • 0.007 (8) slow 0.027 • 0.003 (12) 0.155 • 0.032 (i0) fast (unstripped) 0.026 • 0.005 (I0) slow GLUTAMATE TRANSPORT AND TRANSAMINATION 100- g53 Washout:Alanine, Glutamate (after loadingfrom lumen) Alanine Kexit (unstripped)-0.165rain-1 50- T c~ E ti0. 058 rain-1 | 10' ~ Kexti=O O.3mi 6 nq ~ _ o ,r o ' ' zb ' Minsofwashout Fig. i. Vascular appearance of glutamate and alanine during washout from the luminally preloaded epithelium of R. ridihunda small intestine. Both experiments were carried out on the same animal~ and in each the epithelium was loaded from the lumen with 1 mM amino acid until the rate of vascular appearance reached a steady state; after the removal of solute from the lumen~ washout was followed for the subsequent 30 (alanine) or 35 (glutamate) min. unstripped) = 0,165 rain-I; glutamate exit is monophasic and slow~ with Kexit = 0.036 rain -I, Wash-in to the epithelium from the vascular bed The rate constants for amino acid entry into the epithelium from the vascular bed across the basolatera] membrane (Table 1) indicate that alanine entry and exit are remarkably similar~ there being no significant difference between Kexit (fas% unstripped) and Kentry. In contrast~ Ke l.i.l .l y. for glutamate is one order of magnitude greater than Kexit ~ indicating that glutamate transport is highly asymmetric. 85# BOYD & PERRING Exit from the vascularly preloaded epithelium Alanine washout from the vascularly preloaded epithelium (mean Kexit [fast, unstripped] = 0.181 + 0.051 min -t) is similar to exit from t h e e p i t h e l i u m a f t e r loading from the lumen (mean Kexit [fas% unstripped] = 0.150 + 0.008 min-t). However~ glutamate washout from t h e v a s c u l a r l y preloaded epitheJium is described by an initial fast phase of vascular appearance (mean Kexit [fas% unstripped] = 0 . 1 5 5 + 0.032 min -t) and a subsequent slower phase of washout described by rate constant, Kexit (slow) = 0.026 + 0.005 min -t, similar to that describing the slow monoexponential washout of glutamate from the luminally preloaded epithelium. This suggests that the initial rapid phase of washout represents v a s c u l a r a p p e a r a n c e of radioactive alanine (shown to be a major p r o d u c t of g l u t a m a t e m e t a b o l i s m in e v e r t e d rings) derived by t r a n s a m i n a t i o n from glutamate that was originally loaded into the epithelium from the vascular side. The second phase of washout may represent vascular appearance of unmetabolized glutamate. Asymmetric glutamate transport Glutamate exit across the basolateral membrane is extremely slow, while entry is rapid; the biochemical basis for this asymmetry remains unclear. The rates of glutamate (and alanine) entry and exit across the basotateral membrane are not significantly changed by lowering the external Na concentration from 123 mM to 5 mM (Table 2); thus this marked asymmetry in glutamate transport is maintained in the absence of a sodium concentration gradient. Burckhardt et al. (1980) and Sacktor et al. (1981) have demonstrated that potassium is invoJved in energizing glutamate uptake into vesicles prepared from rat-kidney proximal-tubule basolateral and brush-border membranes. Our findings with intact tissue would fit nicely with such a mechanism. Mircheff et al. (1980) have identified both Na-dependent and Na-independent pathways for alanine in basolateral membrane vesicles from rat small i n t e s t i n e ; our e v i d e n c e suggests that the Na-independent pathway predominates in the frog small intestine. The relation between basolateral transport and metabolism The a s y m m e t r y in glutamate basolateral transport is likely to maintain a relatively low plasma concentration of the potentially toxic amino acid glutamate (the concentration of glutamate in the plasma of Rana c a t e s b e i a n a is about 90 pM; Boyd, 1976), while the high intraceilular level of glutamate will favour transamination to alanine. Since the basolateral membrane transport of alanine appears to be symmetrical, both exit and entry being fas% any alanine produced from glutamate will be removed rapidly from the cells of the epithelium, thereby favouring the forward reaction in equation t. Acknowledgement V. S. P. thanks the M.R.C. for a research studentship. GLUTAMATE TRANSPORT AND TRANSAMINATION 855 Table 2. The effect of removing vascular Na (choline substitution) upon rate constants describing glutamate and alan• movements between the epithelium and the vascular bed of R. ridibunda small intestine expressed as a ratio of the rate constant measured in the absence of vascular Na to that measured in the presence of vascular Na Values are means • S.E.M., with parentheses. the number of samples shown in Kentry (-Na+) Kexit (-Na+) Kentry (+Na+) Kexit (+Na+) Alanine 0.8• 1.1• Glutamic a c i d 0.7 • 0.i (3) 1.2• References Boyd CAR (1976) D. Phil. thesis, University of Oxford. Boyd CAR & Parsons DS (1979) Movements of monosaccharides between blood and tissues of vascularly perfused small intestine. J. Physiol. 2879 371-391. Boyd CAR, Cheeseman CI & Parsons DS (1975) Amino acid movements across the wall of anuran small intestine perfused through the vascular bed. J. Physiol. 250, 409-429. Boyer PD (1973) The Enzymes, Vol. IX, Part B, p 464, Academic Press, New York. Brenner M, Niederwieser A & Pataki G (1962) Aminosauren und derivate, in D u n n s c h i c h t - C h r o m a t o g r a p h i e (Stahl E, ed), pp 403-445, Springer-Verlag, Berlin. Burckhardt G, Kinne R, Stange G & Murer H (1980) The effects of potassium and membrane potential on sodium-dependent glutamic acid uptake. Biochim. Biophys. Acta 5999 191-201. Cheeseman CI (1979) Factors affecting the movement of amino acids and small peptides across the vascularly perfused anuran small intestine. J. Physiol. 293, 457-468. Dent CE & Schilling JA (1949) Studies on the absorption of proteins: the amino-acid pattern in the portal blood. Biochem. J. 44~ 318-333. Hanson PJ & Parsons DS (1977) Metabolism and transport of glutamine and glucose in vascularly perfused small intestine of the rat. Biochem. J. 166, 509-519. Mircheff AK, van Os CH & Wright EM (1980) Pathways for alanine transport in intestinal basal lateral membrane vesicles. J. Memh. Biol. 52, 83-92. 856 BOYD & PERRING Sacktor B, Rosenbloom IL, Liang T & Cheng L (1981) Sodium gradient- and sodium plus potassium gradient-dependent L-glutamate uptake in renal basolateral membrane vesicles. J. Memb. Biol. 60, 63-71. Windmueller HG & Spaeth AE (1974) Uptake and metabolism of plasma glutamate by the small intestine. J. Biol. Chem. 249~ 50705079. Windmueller HG & Spaeth AE (1975) Intestinal metabolism of glutamine and glutamate from the lumen as compared to glutamine from the blood. Arch. Biochem. Biophys. 171, 662-672. Wiseman G (1953) Absorption of amino acids using an in vitro technique. J. Physiol. 120, 63-72.