* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Inverted pyramidal neurons in chimpanzee sensorimotor cortex are
Eyeblink conditioning wikipedia , lookup
Neuroanatomy wikipedia , lookup
Neuropsychopharmacology wikipedia , lookup
Development of the nervous system wikipedia , lookup
Optogenetics wikipedia , lookup
Anatomy of the cerebellum wikipedia , lookup
Subventricular zone wikipedia , lookup
Apical dendrite wikipedia , lookup
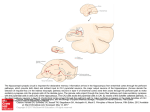
Som atosensory & M otor Research 1999; 16(1): 49 ± 56 Inverted pyramidal neurons in chim panzee sensorim otor cortex are revealed by imm unostaining with m onoclonal antibody SM I-32 HUI-XIN QI 1 , N EERAJ JAIN 1 , TOD D M . PREUSS 2 and JO N H. K AAS 1 1 D epartm ent of Psychology, Vanderbilt U niversity, N ashville, TN 37240; 2 D ivision of B ehavioral B iology, University of Southwestern Louisiana, N ew Iberia R esearch Center N ew Iberia, LA 70560, U SA Abstract We used the m onoclonal antibody SM I-32 to label pyram idal cells of sensorimotor cortex in two chimpanzees. The majority of the pyramidal cells had typical vertically oriented apical dendrites that extended towards the pial surface. A small population of pyramidal cells varied from this orientation, so that the apical dendrites were 20 Æ or more from radial, and were often inverted, extending away from the pial surface. W hen numbers of non-inverted and inverted pyramidal cells were compared, less than 1% were found to be inverted. Key words: Anthropoid prim ate , Pan, som atosensor y cortex , imm unohistochem istr y Introduction Pyramidal neurons in the neocortex of m am m als norm ally extend their apical dendrites towards the pial surface. Van der Loos (1965), however, described cells that deviate from this typical configuration. In a Golgi study of the visual cortex in rabbits, he estim ated that som e 15 ± 20% of pyram idal cells had apical dendrites that deviated 20 Æ or m ore from the norm al orientation, w ith com pletely inverted orientations the m ost frequent of the atypical cells. Small num bers of non-upright pyram idal cells were also recognized in G olgi preparations from rats, cats, and m onkeys. Van der Loos argued that the nonupright pyram idal cells were errors rather than a m orphological adaptation to a functional role. T hus, he concluded that ª the existence of disorientated pyramids m ay be explained by assum ing that, in the turm oil of m igration to, and alignm ent of the cells in, their definitive cortical sites, a few cells, perhaps not surprisingly so, becom e m isaligned.º In support of this hypothesis, larger proportions of inverted pyram idals are described in certain pathological studies that affect cell m igration, such as the reeler m utant m ouse (Landrieu and G offinet, 1981). In addition, inverted pyram ids are thought to be m ore com m on in the deeper layers (Ferrer et al., 1986; van Brederode and Snyder, 1992; Einstein and Fitzpatrick, 1991), w hich develop the earliest. O thers indicate that the functional significance of inverted pyramidals is unknow n, holding out the possibility that they may play a unique functional role (van Brederode and Snyder, 1992). O ne type of unusual pyramidal neuron, the M artinotti cell, with atypically oriented apical dendrites and vertically ascending axons (T Èom b Èol, 1984; Ferrer et al., 1986), m ay well be a specialized type of neuron (Prieto et al., 1994). W hile inverted pyramidals have been observed by a number of investigators in mice, rats, rabbits, cats, and even humans (see Feldman 1984), little is known about the frequency of their occurrence, the locations in cortex where they occur, and the prevalence am ong different species. M ost reports suggest that they are less frequent than indicated by Van der Loos (1965), w ith Parnavelas (1977) estim ating that they represent approxim ately 1% of pyramidal cells in the sensorim otor cortex of rats. T he rarity of observations on inverted pyram idal cells is undoubtedly related to the difficulty of obtaining suitable Golgi m aterial from large am ounts of cortex. This difficulty can now be circum vented by staining pyram idal cells im m unohistochemically. A m onoclonal antibody to neurofilam ent protein, SMI32, labels the cells body and dendrites of a large subset of pyram idal cells in a G olgi-like m anner (Cam pbell and M orrison, 1989, Hof et al., 1995a; 1995b; 1996; N im chinsky et al., 1996; Preuss et al., 1997). In such preparations, it is relatively easy to exam ine the orientations of the apical dendrites of a large number of SM I-32 im m unoreactive neurons. We did this in sections of cortex from the brains of two chim panzees that were processed for SM I-32 Correspondence: Jon H . Kaas, Department of Psychology, Vanderbilt U niversity, 301 W ilson Hall, 111 21st Ave. South, Nashville, TN 37240 USA. Tel: (615) 322 ± 6029; Fax: (615) 343 ± 4342 E-mail: jon.kaas@ vanderbilt.edu 0899 ± 0220/99/01004 9 ± 08 $9.00 1999 Taylor & Francis Ltd 50 Hui-X in et al. antibody as part of a m ore extensive study of the architecture of sensorim otor cortex. The results indicated that inverted and other non-upright pyramidal cells exist am ong the SMI-32 im munoreactive cells of chimpanzee sensorim otor cortex, but they are rare. M ethods M aterial from the brains of two adult chim panzees (Pan troglodyt es ) were obtained from the N ew Iberia Research Center, N ew Iberia, LA and Yerkes Regional Primate C enter, Atlanta, GA. Both anim als died of natural causes and were perfused within ten to twenty m inutes of death. T he chim panzees were perfused transcardially with phosphate buffered saline (PBS, pH 7.4), followed by 4% paraform aldehyde in 0.1 M phosphate buffer, and subsequently with 4% paraform aldehyde with 10% sucrose. T he brains were blocked and post fixed in 4% paraform aldehyde and 10% sucrose in phosphate buffer for two weeks, and further cryoprotected by im m ersion in buffered 20% and 30% sucrose solutions. T he blocks of tissue containing sensorim otor cortex were cut perpendicular to the central sulcus (Fig. 1) or in a parasagittal plane into 50 m m thick sections w ith a freezing m icrotom e. Every tenth section was reacted for SM I-32 prim ary antibody (Sternberger M onoclonals, Inc.) at a 1: 2000 dilution for 40 ± 48 h at 4 Æ C. Further details of processing procedures have been described by Preuss et al. (1997). Remaining series of the brain sections were processed with other histochem ical procedures useful for identifying architectonic boundaries (see Q i et al., 1997). T hese sections were used to identify boundaries of cortical areas 4, 3a, 3b, 1 and 2 in the present study. Brain sections were exam ined with a Zeiss Axioskop m icroscope at 400X m agnification and pyram idal cells were identified. In order to estim ate the F I G U R E 1. A schematic drawing of the dorsolateral view of the left hemisphere of a chim panzee brain. The solid line indicates the plane of sectioning perpendicular to the central sulcus and corresponds to the section shown in Figure 2A. cs, the central sulcus extends to the midline; M , medial; R, rostral. population of SM I-32 im m unoreactivity (SM I-32-ir) pyramidal cells that were inverted, a 10 m m 3 10 m m grid was superim posed on layers III, V, and VI, and pyram idal cells were counted within the grid. Counts were m ade in a total of 1057 sem irandom ly selected sam ple fields through the som atosensory and m otor cortex. We classified cells as atypical pyram idal cells if they had triangular cell bodies and long stout apical dendrites that deviated m ore than 20 Æfrom radial orientation (follow ing Van der Loos, 1965). W ithin this class of atypical pyram idal cells, we also distinguished a subclass of inverted pyram idal cells, which had apical dendrites directed toward deeper layers of cortex or the white m atter, and basal dendrites that ascended toward the superficial layers. D igital im ages were acquired for the purpose of illustrating inverted pyram idal cells by using a M icrolumina L eaf digital cam era m ounted on a N ikon E800 m icroscope. T he digitized im ages were adjusted for brightness and contrast using Adobe Photoshop, but they were not otherwise altered. Results T he SM I-32 antibody labeled cell bodies and dendrites of neurons throughout sensorim otor cortex. T hese cells were concentrated in a deep band corresponding to layer V and a superficial band of neurons in the inner half to two-thirds of layer III. Som e neurons in other layers were labeled as well. T he great m ajority of these labeled neurons were clearly pyram idal cells, although nonpyram idal cells were occasionally labeled. Large num bers of neurons were labeled in area 4 (Fig. 2A) and in area 3a, while fewer area 3b neurons were labeled. Areas 1 and 2 (Fig. 3A) had more SM I-32 im m unoreactive neurons than area 3b, but fewer than areas 4 and 3a. T hese results w ill subsequently be described in m ore detail (see also Q i et al., 1997). O f the great num ber of pyram idal cells that were exam ined in these fields, the vast m ajority were typical pyram idal cells w ith the long axis of the cell body oriented perpendicular to the brain surface. N evertheless, pyram idal cells with atypical orientations were occasionally observed. The m ost com m on of the atypical pyram idal cells were the inverted pyramidal cells, such as those shown in Fig. 2B, 2E, 3B and 3C. Other atypical pyram idal cells were not so perfectly inverted, although they fell within our classification of inverted. Thus, the apical dendrite of the neuron in F ig. 2D coursed at a downward angle about 40 Æ from radial (i.e., perpendicular to the pia). Another exam ple of a poorly oriented but inverted pyramidal neuron is illustrated in Fig. 2C. The inverted pyram idal cells were m ost often found in layer VI, but they were present in layers III and V as well. T he widths of cell bodies ranged from 10 ± 25 m m , and apical dendrites could be traced as far as 400 m m . T he thin axons of these neurons were Inverted pyramidal cells 51 F I G U R E 2. Photom icrographs of m otor cortex stained with SM I-32 antibody. The pial surface is towards the top in all panels. (A) A low magnification photomicrograph of a parasagittal section through area 4. Roman num erals indicate the cortical layers. The arrow indicates an inverted pyram idal cell shown at higher magnification in (B). (B) An example of an inverted pyramidal cell in layer III of area 4. (C) An inverted pyram idal cell in layer III of premotor cortex. A pyramidal cell with an atypical orientation is located to the left of the arrow. (D) A poorly oriented inverted pyramidal cell in layer V of area 4. (E) An inverted pyramidal cell in layer VI of area 4. The locations of cells (B), (C), (D) and (E) are shown in the inset. Scale bars: 200 m m in (A); 50 m m in (B), (C), (D), and (E). Arrows indicate the inverted pyramidal cells. ad, apical dendrite. 52 Hui-X in et al. F I G U R E 3. Photomicrographs of somatosensory areas stained with the SMI-32 antibody. (A) A low magnification photomicrograph through area 2. Roman numerals denote the six cortical layers. The arrow indicates an inverted pyramidal cell shown at higher magnification in (B). (B) An example of an inverted pyramidal cell (arrow) in layer VI of area 2. (C) An inverted pyramidal cell in layer VI of area 3b. (D) Location of the cells shown in (B) and (C). Scale bars: 200 m m in (A); 50 m m in (B) and (C). ad, apical dendrite. Inverted pyramidal cells som etim es apparent, but could be traced for only short distances. Van der Loos (1965) also observed such atypically oriented pyram idal cells, including those with apical dendrites extending towards the pial surface but at an angle. Since m any pyram idal cells are not perfectly oriented in the radial axis, Van der Loos (1965) classified pyram idal neurons as m isoriented if they reached the arbitrary criterion of being rotated 20 Æ or m ore from radial. Such a neuron is shown in Fig. 4A with an apical dendrite that extended at a 45 Æ angle from vertical. In contrast, the neuron in Fig. 4B, started at a slight angle off the radial, but changed its course to becom e vertically oriented. This m ay be regarded as a sm all error, which did not m eet our criteria for an atypically oriented pyram idal cell. T he cell in Fig. 4C also m ade a correction, but m uch of the proxim al portion 53 of the apical dendrite coursed away from the cell body at an angle of more than 20 Æ . In a final exam ple (Fig. 4D ), the apical dendrite coursed along in a horizontal plane parallel to the pial surface. T hus, atypical pyram idal cells had a range of orientations, and the estim ated frequency of such cells will depend on the criteria used to identify them . O f 1977 identified pyram idal neurons, 12 (0.61%) were classified as inverted, in that the apical dendrite curved from the som a at an angle below the horizontal plane. T he great m ajority of inverted pyramidal cells was located in cortical layer V I (Table 1). T hus, 1 of 1004 identified pyram idal cells was classified as inverted in layer III (0.10% ), 1 of 527 pyramidal cells was inverted in layer V (0.19%), and 10 of 446 identified pyramidal cells were inverted in layer V I (2.24%). T hus, it appears that for the F I G U R E 4. Photomicrograph illustrating atypically oriented pyramidal cells. (A) In the cell denoted by the arrow, the long axis of the cell body and apical dendrite deviates 45 Æfrom radial. (B) A ``regular’’ pyramidal cell (arrow). The cell body started at an angle slightly off radial, but the apical dendrite corrected its course to radial. (C) A ``regular’’ pyramidal cell in which the apical dendrite curved to make a correction, but much of the proximal portion of the apical dendrite coursed away from the cell body at an angle of more than 20 Ê. (D) An exam ple of a horizontal pyramidal cell (arrow) in which the apical dendrite traveled in the plane parallel to the pial surface. Scale bars: 50 m m . 54 Hui-X in et al. TA B L E 1. Laminar distribution of inverted pyramidal cells in sensorimotor cortex M otor cortex Layer III Layer V Layer VI Total Som atosensory cortex Sensorimotor cortex TP IP IP/TP TP IP IP/TP TP IP IP/TP 576 332 264 1172 1 1 8 10 0.17% 0.30% 2.94% 0.85% 427 194 172 793 0 0 2 2 0% 0% 1.15% 0.25% 1003 526 436 1965 1 1 10 12 0.10% 0.19% 2.24% 0.61% Abbreviations: IP, inverted pyramidal cells; TP, typical pyramidal cells. subpopulations of SMI-32 positive pyram idal cells, less than one percent are inverted. Additional counts were m ade through the sensorim otor area in order to estim ate the percentage of atypically oriented pyram idal cell, w hich included all pyramidal cells w ith apical dendrites that deviated m ore than 20 Æfrom the vertical, including inverted pyramidal cells. Am ong a total of 704 identified pyramidal cells, 18 (2.56% ) were classified as atypical (Table 2). O f these, 2 of 396 cells were classified as atypical in layer III (0.51% ), 4 of 166 in layer V (2.41%), and 12 of 142 in layer VI (8.45% ). horizontal pyram idal cells with apical dendrites that are oriented directly perpendicular to the plane of the section (i.e., directly toward or away from the observer) would probably not be identified as pyram idal cells. Atypical pyram idal cells m ay thus be as m uch as twice as com m on as indicated by the frequency of recognizable atypical cells. To our knowledge, this is the first imm unocytochem ical study of atypical pyram idal cells, and the first report of such cells in chim panzees. O ur observations illustrate the value of the SM I-32 antibody as a tool for studying pyram idal cell m orphology, which results from its relatively com plete, G olgi-like staining of the pyram idal cell bodies and dendrites. SM I-32 labels m any, but not all, pyramidal cells; it leaves unlabeled m ainly sm aller pyramidal and nonpyram idal cells. Presum ably, the latter cells are unstained because they express little neurofilam ent protein, or express neurofilam ent proteins that lack the epitope recognized by SM I-32 m onoclonal antibody (see Cam pbell and M orrison, 1989). In any case, the labeling favors large pyram idal neurons, especially those that have long projections (C am pbell and M orrison, 1989). Previous observations of inverted and atypically oriented pyram idal cells were based alm ost exclusively on G olgi im pregnation techniques (an exception being de Lim a et al., 1990). Like SM I-32 im m unocytochem istry, the Golgi technique also labels a subset of cells, although perhaps a random subset with respect to the neuronal type and size. Even with the Golgi technique, there have been few system atic studies of the frequency of atypical Discussion We used the SMI-32 antibody to label a subset of pyramidal cells in the sensorim otor cortex of two chim panzees. M ost pyram idal cells had radially oriented apical dendrites extending towards the pial surface, but neurons w ith apical dendrites of other orientations were occasionally obser ved as well. T hese unusual orientations ranged from a modest 20 Ædeviation from vertical near the cell body with subsequent corrections to near vertical, to the m ore com m on com pletely inverted pyram idal cells. According to our estim ate, inverted pyram idal cells were less than 1% of total SM I-32-ir pyram idal cells. However, the total proportion of atypically oriented pyramidal cells with unusual orientation (those 20 Æ or m ore off the vertical; Van der Loos, 1965) was over 2% and constituted over 8% of the pyram idal cells in layer VI. T he actual num ber of atypically oriented pyramidal cells is likely to be higher than this since TA B L E 2. Laminar distribution of atypically oriented pyramidal cells in sensorimotor cortex M otor cortex Layer III Layer V Layer VI Total Som atosensory cortex Sensorimotor cortex TP IP IP/TP TP IP IP/TP TP IP IP/TP 246 109 89 444 2 2 8 12 0.81% 1.80% 8.25% 2.63% 148 53 41 242 0 2 4 6 0% 3.64% 8.89% 2.42% 394 162 130 686 2 4 12 18 0.51% 2.41% 8.45% 2.56% Abbreviations same as Table 1. Inverted pyramidal cells pyramidal cells. It has been reported that inverted and atypically oriented pyramidal cells are present in the cortices of m ice, rats, dogs, cats, m onkeys, and hum ans (e.g., Van der Loos, 1965; G lobus and Scheibel, 1967; W illiam s et al., 1975; Parnavelas et al., 1977; Ferrer et al., 1986; M iller, 1988), that inverted pyramids are one of the m ost com mon of the atypically oriented types (Van der Loos, 1965), and that they are m ore frequent in deeper layers (Van Brederode and Snyder, 1992; Ferrer et al., 1986a,b, 1987; E instein and Fitzpatrick, 1991) and in abnorm al cortex (W illiam s et al., 1975; Landrieu and G offinet, 1981; Prieto et al., 1994). Van der Loos (1965) appears to have made the m ost serious attem pt to estimate the proportion of atypically oriented pyramidal neurons, and yet his estim ate of 18% in rabbit visual cortex (based on 33 atypical out of 183 pyramidal cells) is higher than subsequent estim ates of 5% in rabbits (G lobus and Scheibel, 1967) and 1% in rats (Parnavelas et al., 1977). The proportion of such cells m ay var y across species and cortical areas, but it seems fair to conclude, based both on previous reports and on the present study, that atypically oriented and inverted pyram idal cells are generally uncom m on, perhaps 1± 3% , although they m ay constitute a m uch higher proportion of the pyram idal cells in layer VI, on the order of perhaps 10%. If these unusual neurons reflect errors in developm ent, it seems fair to ask what the consequences for neural processing m ight be. A 1± 5% error rate in the development of norm al dendritic orientation m ay not have m uch im pact on neural networks. In addition, given the great plasticity of the developing brain, and the evidence that m ost of the atypically oriented neurons have axons that course norm ally (Einstein and Fitzpatrick, 1991; M iller, 1988; D e Lim a et al., 1990), many or m ost of the abnorm al neurons m ay have adopted partially functional roles. In their intracellular recording of layer V I pyram idal cells from slices of rat sensorim otor cortex, van Brederode and Snyder (1992) did not find any differences between the intrinsic electrical properties of the regular and irregularly oriented pyram idal cells. In addition, the possibility rem ains that atypically oriented neurons represent functional subtypes, although they are uncom m on, especially in layers III and V. If inverted pyram idal cells represent developm ental errors, as proposed by Van der Loos (1965), then one m ight expect large-brained and sm all-brained species to vary in the proportion of errors. The rate of neuorblast m igrations and the generation of cortical layers is m uch m ore rapid in brain in rats and m ice than in m onkeys (see Rakic, 1977), and rapid traffic along m igratory paths m ight generate m ore disoriented neurons. In contrast, if inverted pyram idal cells represent a specialized cell class with a specific functional role, they m ight be m ore frequent in the large hum an and chim panzee brains, where there m ay be m ore morphological specialization of neu- 55 rons (Parnavelas et al., 1977; M eyer, 1987). O ur results, however, suggest that inverted pyram ids are no more frequent in chimpanzees than in rabbits and rats. W hether the atypically oriented pyram idal cells are a developm ental error or a function subtype rem ains to be determ ined. Acknowledgm ent Supported by N IH G rant N S16 446 and by USLN IRC . References C A M P B E L L , M .J., and J.H. M O R R IS O N (1989) Monoclonal antibody to neurofilament protein (SM I-32) labels a subpopulation of pyramidal neurons in the human and monkey neocortex. J Com p N eurol 282 : 191± 205. D E L IM A , A.D., T. V O IG T, and J.H. M O R R IS O N (1990) M orphology of the cells within the inferior temporal gyrus that project to the prefrontal cortex in the macaque monkey. J C omp Neurol 296 : 159 ± 172. E I N S T E I N , G., and D. F I T Z PAT R I C K (1991) Distribution and morphology of area 17 neurons that project to the cat’s extrastriate cortex. J Com p N eurol 303 : 132 ± 149. F E L D M A N , M .L. (1984) Morphology of the neocortical pyramidal neuron. In A. P E T E R S , and E. G. JO N E S eds: Cerebral Cortex, Vol. 1, Cellular Components of the Cerebral Cortex , pp. 123 ± 200. New York: Plenum Press. F E R R E R , I., I. F A B R E G U E S , and E. C O N D O M (1986a) A Golgi study of the sixth layer of the cerebral cortex. I. The lissencephalic brain of Rodentia, Lagomorpha, Insectivora and Chiroptera. J Anat 145 : 217± 234. F E R R E R , I., I. F A B R E G U E S , and E. C O N D O M (1986b) A Golgi study of the sixth layer of the cerebral cortex. II. The gyrencephalic brain of Carnivora, Artiodactyla and Primates. J Anat 146 : 87± 104. F E R R E R , I., I. FA B R E G U E S , and E. C O N D O M (1987) A Golgi study of the sixth layer of the cerebral cortex. III. Neuronal changes during norm al and abnormal cortical folding. J Anat 152 : 71± 82. G L O B U S , A., and A.B. S C H E IB E L (1967) Pattern and field in cortical structure: the rabbit. J Comp Neurol 131 : 155 ± 172. H O F, P.R., and J.H. M O R R I S O N (1995a) Neurofilam ent protein defines regional patterns of cortical organization in the macaque m onkey visual system : a quantitative imm unohistochemical analysis. J Com p Neurol 352 : 161± 186. H O F, P.R., E.J. M U F S O N , and J.H. M O R R I S O N (1995b) Human orbitofrontal cortex: cytoarchitecture and quantitative imm unohistochemical parcellation. J Com p N eurol 359 : 48 ± 68. H O F, P.R., L.G. U N G E R L E ID E R , M .J. W E B S T E R , R. G ATTA S S , M.M . A D A M S , C.A. S A I L S TA D , and J.H. M O R R I S O N (1996) Neurofilam ent protein is differentially distributed in subpopulations of corticocortical projection neurons in the macaque m onkey visual pathways. J Com p Neurol 376 : 112 ± 127. L A N D R IE U , P., and A. G O F F I N E T (1981) Inverted pyram idal neurons and their axons in the neocortex of reeler mutant mice. Cell Tissue Res 218 : 293 ± 301. M E Y E R , G. (1987) Forms and spatial arrangement of neurons in the primary m otor cortex of m an. J C om p Neurol 262 : 402 ± 428. M IL L E R , M .W. (1988) M aturation of rat visual cortex: IV. The generation, m igration, morphogenesis, and connectivity of atypically oriented pyramidal neurons. J Com p Neurol 274 : 387± 405. 56 Hui-X in et al. N I M C H I N S K Y, E.A., P.R. H O F, W.G. YO U N G , and J.H. M O R R IS O N (1996) Neurochemical, morphologic, and laminar characterization of cortical projection neurons in the cingulate m otor areas of the macaque monkey. J Com p Neurol 374 : 136 ± 160. PA R N AV E L A S , J.G., A.R. L IE B E R M A N , and K.E. W E B S T E R (1977) Organization of neurons in the visual cortex, area 17, of the rat. J Anat 124 : 305 ± 322. P R E U S S , T.M ., I. S T E P N IE W S K A , N. JA IN , and J.H. K A A S (1997) Multiple divisions of macaque precentral m otor cortex identified with neurofilam ent antibody SM I-32. B rain Res 767 : 148 ± 153. P R IE T O , J.J., B.A. P E T E R S O N , and J.A. W I N E R (1994) M orphology and spatial distribution of GABAergic neurons in cat primary auditory cortex (AI). J C omp Neurol 344 : 349 ± 382. Q I , H.-X., N. JA I N , T.M. P R E U S S , and J.H. K A A S (1997) Histochem ical organization of somatosensory area 3b and surrounding cortex in chimpanzees. Soc. Neurosci. Abstr. 23 : 1007. R A K I C , P. (1977) Prenatal development of the visual system in rhesus monkey. Phil Trans R Soc Lond (B ) B iol Sci 278 : 245 ± 260. T OÈ M B OÈ L , T. (1984) Layer VI cells. In A. P E T E R S , and E. G. JO N E S eds: C erebral C ortex, Vol. 1, C ellular C om ponents of the Cerebral C ortex . pp. 479 ± 519. New York: Plenum Press. VA N B R E D E R O D E , J.F., and G.L. S N Y D E R (1992) A com parison of the electrophysiological properties of m orphologically identified cells in layers 5B and 6 of the rat neocortex. Neuroscience 50 : 315 ± 337. VA N D E R L O O S , H. (1965) The ª improperlyº oriented pyramidal cell in the cerebral cortex and its possible bearing on problem s of neuronal growth and cell orientation. B ull Johns H opkins H osp 117 : 228 ± 250. W I L L IA M S , R.S., R.J. F E R R A N T E , and V.S. C AV I N E S S , Jr. (1975) Neocortical organization in hum an cerebral malformation: A Golgi study. Soc Neurosci A bs 1 : 776.