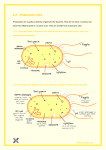
* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Cell Division Studies of Escherichia Coli: Expression and Protein
Epigenetics in stem-cell differentiation wikipedia , lookup
Epigenetics of human development wikipedia , lookup
Cre-Lox recombination wikipedia , lookup
Designer baby wikipedia , lookup
Protein moonlighting wikipedia , lookup
DNA vaccination wikipedia , lookup
History of genetic engineering wikipedia , lookup
Gene therapy of the human retina wikipedia , lookup
Point mutation wikipedia , lookup
Site-specific recombinase technology wikipedia , lookup
Mir-92 microRNA precursor family wikipedia , lookup
Polycomb Group Proteins and Cancer wikipedia , lookup
No-SCAR (Scarless Cas9 Assisted Recombineering) Genome Editing wikipedia , lookup
Therapeutic gene modulation wikipedia , lookup
Artificial gene synthesis wikipedia , lookup
Louisiana State University LSU Digital Commons LSU Historical Dissertations and Theses Graduate School 1988 Cell Division Studies of Escherichia Coli: Expression and Protein Localization of Cell Septation Gene, FtsA. Chong Chon Younghae Louisiana State University and Agricultural & Mechanical College Follow this and additional works at: http://digitalcommons.lsu.edu/gradschool_disstheses Recommended Citation Younghae, Chong Chon, "Cell Division Studies of Escherichia Coli: Expression and Protein Localization of Cell Septation Gene, FtsA." (1988). LSU Historical Dissertations and Theses. 4564. http://digitalcommons.lsu.edu/gradschool_disstheses/4564 This Dissertation is brought to you for free and open access by the Graduate School at LSU Digital Commons. It has been accepted for inclusion in LSU Historical Dissertations and Theses by an authorized administrator of LSU Digital Commons. For more information, please contact [email protected]. INFORMATION TO USERS The most advanced technology has been used to photo graph and reproduce this manuscript from the microfilm master. UMI films th e text directly from the original or copy submitted. Thus, some thesis and dissertation copies are in typewriter face, while others may be from any type of computer printer. The quality of th is reproduction is dependent upon the quality of the copy submitted. Broken or indistinct print, colored or poor qu ality illu stratio n s and photographs, print bleedthrough, substandard margins, and improper alignment can adversely affect reproduction. In the unlikely event th at the author did not send UMI a complete m anuscript and there are missing pages, these will be noted. Also, if unauthorized copyright m aterial had to be removed, a note will indicate the deletion. Oversize m aterials (e.g., maps, drawings, charts) are re produced by sectioning th e original, beginning at the upper left-hand corner and continuing from left to right in equal sections w ith small overlaps. Each original is also photographed in one exposure and is included in reduced form at the back of the book. These are also available as one exposure on a standard 35mm slide or as a 17" x 23" black and w hite photographic p rin t for an ad d itio n al charge. Photographs included in the original m anuscript have been reproduced xerographically in th is copy. H igher quality 6" x 9" black and w hite photographic p rin ts are available for any photographs or illustrations appearing in this copy for an additional charge. Contact UMI directly to order. UMI University M icrofilm s International A Bell & Howell Information C om pany 300 North Zeeb Road, Ann Arbor, Ml 48106-1346 USA 313/761-4700 800/521-0600 Order N u m b er 8904533 Cell division stu dies o f Escherichia coli: E xp ression and p rotein lo ca lization o f cell sep tation gene, fts A Younghae, Chong Chon, Ph.D. The Louisiana State University and Agricultural and Mechanical Col., 1988 UMI 300 N. Zeeb Rd. Ann Arbor, MI 48106 Cell division studies of Escherichia c o l i : Expression and protein localization of cell septation gene, fisA. A Dissertation Submitted to the Graduate Faculty of the Louisiana State University and Agricultural and Mechanical College in partial fulfillment of the requirements for the degree of Doctor of Philosophy in The Department of Microbiology by Younghae Chong Chon B.P., Ewha Womans University, 1976 M.S., Louisiana State University, 1984 August 1988 ACKNOWLEDGEMENTS I would like to express my deepest appreciation to Dr. Randall Gayda for all of the advice and support he has extended to me over the past few years. His discussionsand insights have been great inspirations and sources of motivation throughout the course of this investigation and invaluable to the completion of this degree. I would also like graduate committee, Marion Socolofsky Dr. to express gratitude to the members Eric Achberger, and Dr. Ding Shih Dr. for Michael their of my Orlowski, timely advice Dr. and education in general. Special thanks go to Dr. Ronald Siebeling for advice and his students for assistance in antibody preparations and to Mrs. Margaret C. Henk for excellent technical advice in electron microscopy. Thanks also to the graduate students and remaining faculty and staff of the Department of Microbiology for making my stay in Louisiana a fine experience. Finally, daughter, work. I would Hyaejeong for like to thank my husband, Inheung, their love, understanding and support and in my TABLE OF CONTENTS page ACKNOWLEDGEMENTS ........ ii TABLE OF CONTENTS........ iii LIST OF TABLES ................................................... vi LIST OF FIGURES................................................. vii ABSTRACT .................................................... ix INTRODUCTION ..........................*........................... 1 LITERATURE REVIEW I. Identification of cell division genes....................... 4 II. Roles of penicillin binding proteins in morphogenic cycle. .......................... 12 III. Periseptal annuli andpotentialdivision sites................ 16 IV. Preliminary studies on the regulation of ftsQAZ gene expression....................................... 18 MATERIALS AND METHODS I. Bacterial strains,plasmids and phages.................... ..21 II. Media and growth conditions............... III. Enzymes and chemicals............................. 21 23 IV. Methods of DNA preparation a. Large-scale plasmid DNA isolation................ ....24 b. Small-scale (rapid) plasmid isolation................ 25 V. General genetic and recombinant DNA techniques............. 26 VI. Bacterial transformations................................. 27 VII. Electrophoretic techniques................................ 28 VIII. Extraction of DNA fragments from agarose g e l s .............. 29 iii IX. Maxicell labelling and plasmid specified proteins.......... 30 X. Plasmid constructions.................................... .31 a. In vivo mini-Mu transposon mutagenesis............... 31 b. Construction of FtsA-LacZ overproducing plasmid,pYC3 c. ........................ 32 Construction of promoter fusion plasmids.............33 XI. Purification of FtsA-LacZ hybrid protein................... 34 XII. Production of FtsZ-LacZ antibodies......................... 35 XIII. Western blot analysis..................................... 36 XIV. Cell fractionation procedures..............................37 XV. Isolation of membrane fractions 0MH,0ML,IM.................. 38 XVI. Immunogold labelling and electron microscopy............... 40 XVII. Preparation of cell extracts for enzyme assays..............41 XVIII. Enzyme assays a. 0-galactosidase assay................................42 b. CAT assay........................................... 42 c. P-lactamase assay....................... XIX. Protein assays. 43 ...................................... 44 XX. Plasmolysis of bacterial cells.............................44 RESULTS I. Isolation of FtsA-LacZ protein fusions......................45 II. Expression of FtsA-LacZ fusion polypeptide in maxicells............................................... 46 III. Cellular localization of the FtsA-LacZ protein............. 46 IV. Overproduction and purification of FtsA-LacZ protein ...................................... 48 iv V. Identification of wild type FtsA protein by Western blots........................................... 49 VI. Cellular localization of FtsA protein A. Western blots of cell fractions.................... ..52 B. Immunogold labelling and electron microscopy a. Wild type cells................................54 b. Plasmolyzed cells..............................55 VII. Investigation of FtsZ gene expression A. Promoter gene fusions and quantitation assays.............................. 56 B. Effects of FtsA, FtsQ, FtsZ, and DnaA on ftsZ expression..................... DISCUSSION 58 .................................................... .60 LITERATURE CITED................................................. 70 VITA .......................................................... 133 LIST OF TABLES Tables Pages 1. Bacterial strains.......................... 82 2. Plasmids and phages........................................ 83 3. Cellular location of FtsA andFtsA-LacZ 4. CAT/B-lactamase specificactivities fusions.............. 84 for different promoter gene fusions..................................... 85 5. FtsZ effects on promoters.................................. 86 6. FtsQ effects on promoters.................................. 87 7. FtsA effects on.promoters.................................. 88 8. DnaA46 mutants effects on promters......................... 89 LIST OF FIGURES Figures 1. pages Locations of coding sequences and promoters within the ddl-envh region of the E. coli chromosome..................... 90 2. Proposed steps in the morphogenesis of E. coli cells................................. 3. 4. Diagram of periseptal annuli in lkyD~cells................ 94 Schematic representation of the expression VeCtOr pGW7 5. 6. 96 The vector pKK232-8 .................................... 98 Restriction maps of pRGC31, Mudll 1734 and mini-Mu insertion locations................... 7. 92 100 Autoradiograph of plasmid-specified polypeptides labelled in UV-irradiated maxicells.............................. 102 8. Separation of membrane fraction by sucrose equilibrium gradient............................................ 104 9. Schematic diagram for construction of expression plasmid, pYC3. 10. ...............................................106 SDS-PAGE profile of proteins for purification of FtsA-LacZ protein from temperature shifted AMC290(Alac) strain containing plasmid pYC3............................. 11. Identification of FtsA 12. Immunodetection of FtsA in AMC290 strain harboring pYC40 or 108 byWestern blot analysis............110 overproducing plasmid pYC3........................................ 112 Figures 13. pages Cellular localization of the FtsA protein by Western blot with FtsA antibodies................................. 114 14. Western blot with FtsA-LacZ antibodies of cell fractions of AMC290...... 15. 116 SDS-PAGE Coomassie blue stain gel profile of AMC290 cellular fractions analysed in immunoblot of Fig. 15....................... 118 16. Western blot of FtsA antibodiespreabsorbed with total membrane of cell fractions of AMC290............................. 120 17. Electron microscopic localization of FtsA in E, coli cells by immunogold labelling.................................... 122 18. Phase contrast light and electron micrographs of plasmolyzed ftsZ84(ts) filaments................................. 125 19. Immunoelectron micrographic localization of FtsA in ftsZ84 filament............................................... 127 20. Localization of FtsA in plasmolyzed ftsZ84 filament by immunoelectron micrograph...................... 21. 129 Promoter fusions of the ftsk-ftsZ regulatory region to the chloramphenicol acetylase gene(Cmr).........................131 ABSTRACT FtsA is a gene essential for cell septation. It has been postulated to have both regulatory and structural functions. To study the structural involvement of the FtsA protein in septum formation, four FtsA-LacZ protein transposon (MudII1734). fusions were constructed using a mini-Mu When maxicells containing either ftsA-lacZ or ftsA specifying plasmids were radioactively labelled and fractionated, FtsA-LacZ fusion protein and FtsA were located in both the membrane and cytoplasmic fractions. located in dividing galactosidase The FtsA-LacZ fusion proteins were also wild activity. type Cell dividing envelope cells fractions by measuring from a fJ- sucrose equilibrium gradient had P-galactosidase activity in outer membraneheavy(OM„) and outer membrane-1ight(0ML ) fractions. To verify the cellular location of native FtsA protein in membrane fractions, an expression plasmid was constructed by placing a ftsA-lacZ gene fusion downstream from a X PL promoter. overproduced and chromatography. purified by The FtsA-LacZ fusion protein was 0-galactosidase affinity column Polyclonal antibodies to this protein were isolated and demonstrated to be specific for the FtsA-LacZ fusion, FtsA protein and B-galactosidase. Cross reactivity to FtsA protein was found in outer membrane fractions, 0M„ and 0ML , and inner membrane fraction of wild type cells. These data suggest that the FtsA protein may be functioning as an inner-outer membrane protein in septation. IX Electron microscopy of thin sections treated with FtsA-LacZ antibodies and immunogold labelling also suggest that the FtsA protein is located in the cell envelope. of FtsA filamentous protein cells by Furthermore, direct identification immuno-electron-microscopy indicates an enrichment septation sites. X of in FtsA normal ' and protein near INTRODUCTION Cell process division that in involves Escherichia the coli coordination physiological growth processes including mass and volume, is and a biological regulation of several increases in cell length, initiation of DNA replication, replicated chromosomes. complex and segregation of The final steps in cell division are the coordinated invagination of the three envelope layers(inner membrane, murein, outer membrane) at the cell center (septation) and then physical separation of the newly formed daughter cells. Progress has been made in understanding cell division at the genetic level. Conditional lethal mutants that cannot form septa have been isolated and referred to as filamentation temperature-sensitive (fts) mutants. septum are map. A number of genes involved in the formation of the clustered at the 2 minute region on the E. coli genetic Based on phenotypic properties, these genes have been postulated to have essential roles in the initiation of septum formation (ftsl and ftsQ) and later invagination stages (ftsk and ftsl). have been cloned and the DNA sequenced. confirmed genetic genes(ftsQ,ftsA,ftsZ) studies that are closely linked, These genes DNA sequence analysis has three of that there these fts is no strong transcription terminator between the genes, and that promoters exist upstream of each of the genes. However, information concerning the regulatory and morphogenetic functions of these genes in the septation process at the molecular level is only starting to be accumulated. At present the biochemical roles of the FtsQ, FtsA, and FtsZ proteins in septum formation are not known. 1 2 Microscopic studies of cells and physiological analyses of cell division mutants morphogenetic division division, have suggested pathway. mutants, the that Phenotypic such as cell percentage the properties shape, of septation of the persisting process is conditional amount of constrictions a cell residual at the restrictive temperature and the pattern of division recovery after a shift back to the permissive temperature have suggested that the cell division genes are activated in the order FtsZ-FtsQ-FtsA-FtsI. example, conditional lethal nonpermissive temperature constrictions at mutants do not potential cell of divide ftsA but division when filament sites. shifted to with partial Thus, protein appears to function after septation initiation. For the FtsA The ordered functions of these cell division genes may be controlled at the level of new proteins. transcription or the activation of previously existing It is probable that both events occur but the current evidence strongly favors the protein activation model as the primary mechanism which commits a cell to septum formation. In this thesis, synthesis and I attempt to verify the hypothesis that the invagination of surface layers constituting the new septum require the formation of a structure which has been called the "septalsome". The septalsome is a multienzyme-protein complex in which the known septation proteins (FtsZ, FtsQ, FtsA and Ftsl) and perhaps others would initiate and control the septation steps. This septalsome would contain both specific peptidoglycan synthesizing and modifying enzymes plus envelope structuring proteins essential for 3 septation. This organelle would thus ensure the precise coordination of the enzymatic activities and localization of the septation proteins within the cell envelope. In this study, I have focus primarily upon the FtsA protein as a potential septalsome protein. Since the amount of FtsA within a cell is small, the construction of FtsA fusion proteins were necessary. The location of these fusion proteins in dividing wild type cells was accomplished by assaying 0-galactosidase activities of the FtsA-LacZ fusion proteins investigate in soluble and membrane location of authentic fraction of cells. FtsA protein, a To plasmid which overproduced the FtsA-LacZ protein was constructed and fusion protein was purified and injected into rabbits for production of polyclonal antibodies. Western blotting experiments were conducted with these antibodies on cell membrane fractions. Direct visualization of FtsA protein at septation sites was done by immunoelectron microscopy. In addition, in various promoter fusion plasmids were constructed attempts to investigate the regulation of the cell division genes, ftsk and ftsZ. Literature Review I. Identification of cell division genes. A number of genes essential for cell division in Escherichia coli have been mapped to the 2 min region of the genetic map(36,3). These genes have been mainly identified as temperature sensitive(ts) lethal mutations, so called fts(filamentation temperature sensitive) phenotype, that result in multi-nucleated filamentous growth and eventual cell death at the nonpermissive temperaturedl,77,94,120). The ftsZ(sulB) gene and two neighboring cell division genes, ftsQ and ftsA in this 2 min region(94,96,109,129,130) are part of a large cell envelope-cell division gene cluster (Fig. 1). That is, in addition to fts genes this 2 min region encodes for murein genes( 8 genes) which specify enzymes required for the peptidoglycan synthesis of the cell wall, cell permeability-cell separation gene, envA(7), and the secA gene which is essential in protein export across the cytoplasmic membrane(90). This close clustering of cell envelope genes and cell division genes suggests possible coordinated interactions among the gene and gene products that result in septum formation. The peptidoglycan synthesis and ftsZ, ftsQ, ftsA and ftsl gene in this cluster encode proteins that probably are required to initiate, complete a cell septum. form and They have been hypothesized to act in a morphogenic pathway(9,Fig. 2) although little is known about their specific functions or biochemical activities in septum formation. FtsZSk is a temperature sensitive(ts) mutant that at the nonpermissive temperature forms multinucleated nonseptated filaments lacking any persisting or newly initiated septal constriction(77). When double mutants that were 4 both temperature sensitive for 5 elongation(rodA) and septation were analyzed(9), the results indicated that the formation of any septum-like constriction required expression of the FtsZ gene product. That is, FtsZ acts earlier than FtsQ, FtsA, or FtsI(PBP3). synthesis(127) revealed that was be found to (septum sites) nonconstricting Analyses ftsl ftsZ84 new peptidoglycan radioactive DAP(Diaminopimellic incorporated of of near newly initiated acid) constriction mutant filaments, but was not found mutant filaments. In addition,a in recent morphogenic and physiological study(113), investigating the formation of septal constrictions and the amount of cell division of ftsZ,ftsQ,ftsA, and ftsl mutants after a temperature shift from 28°C to 42°C, has suggested that the functioning ofthese cell division genes is in the order FtsZ- FtsQ- FtsA,Ftsl. Genetic demonstrated and biochemical that the FtsZ(SulB) evidences gene (45,46,56,57,58) product SOS(DNA damaged) induced cell filamentation. is the have target of SulA(SfiA) synthesis is induced following DNA damage as are DNA repair enzymes that are regulated by the LexA-RecA regulatory circuit(86,71). SulA protein is labile with a half-life of 3 min in wild type cells(64). In a Ion mutant(101,180) SulA is more stable with a half-life near 20 minutes, which enhances its inhibitory effects and leads to filamentation and death of the Ion irradiation(56,58). mutant after SOS induction such as UV SulB(sfib) mutations (like sulA(sfiA) mutations) were isolated as second site mutations that confer UV resistance to a Ion mutant. The suIB locus was found to be identical to ftsZ gene locus(45,73,64). The hypothesis that FtsZ protein is supported by the findings that isthe target of SulA the overproduction of FtsZ 6 can suppress the SOS-induced lethal filamentation in Ion mutants(75), and that biochemical studies suggest direct interactions between the FtsZ protein and SulA proteins because the half-life of radioactively labelled SulA in maxicells was increased when cells contained the wild type ftsl gene but not sulB mutation(65). The inhibition of cell septation by SulA interacting with FtsZ is reversible in wild type cell since SOS-induced filaments eventually divide even in the absence of de novo protein synthesis(80). It has been hypothesized that a possible role of FtsZ protein is as an element of the cell division apparatus to which the cell transmits information about growth conditions(67). This role for FtsZ is based on studies with a ftsZ(sfiB114) mutant. unable to interact with SulA(64) but it This mutant is had a longer cell septation delay( 50 to 60 min.) compared with wild type (20 min) after a shiftup from glucose minimal medium to complex media, and it had a shortened cell septation delay (10 to 16 min) after a nutritional pulse (a shift up to complex media followed rapidly by a return to glucose minimal medium). When the level of ftsZ gene expression was increased, minicell formation and increased septation that results in slightly smaller cells have been observed. Ward and Lutkenhaus(123) found that just a two to seven-fold increase of FtsZ synthesis induces polar divisions producing minicells. These minicells were not formed at the expense of as normal divisions, they are in a minh minicell producing mutants, but were the result of increased septation. The small cell size of cells containing multicopy-plasmids specifying ftsZ appears to be because medial septal invaginations are initiated earlier in 7 the cell division cycle. However, specifying multicopy-plasmid into the introduction of a ftsZ minb mutants results in just increased polar division producing more minicells with normal medial divisions(123). This observation suggests between the FtsZ and minB locus products. that FtsZ protein is involved in potential(medial) division sites. the possible interactions These findings suggest maturation stage of the The FtsZ-induced production of minicells was suppressed in cya mutant(27). cya mutant cell phenotype is short rods because cAMP-CRP(Catabolie necessary for longitudinal cell growth. with earlier observations(55) that Regulatory Protein) is This finding is consistent introduction of cya or crp mutation into a minb minicell strain suppresses minicell production. The function of cAMP-CRP in minicell formation is postulated to be between the division machinery and PBP2(penicillin binding protein 2). Minicell strains are deficient in PBP2.( see Sect2 for role PBP2). The ftsZ(sulb) gene has been subcloned and sequenced (95,129) and identified as a 45 Kdal protein that is membrane associated(53). Ward and Lutkenhaus have purified the FtsZ protein and have made antibodies to it (75). They found that when cells were screened by Western blotting a FtsZ analogue was antigenically conserved in all tested procaryotes both Gram-negative and Gram-positive. Only closely related enterobacteriaceae, however, hybridized with an E. coli ftsZ DNA probe by Southern blotting. of Furthermore,the induction minicells phenotype was observed in other strains transformed 8 with multicopy-plasmids containing ftsZ gene, demonstrating that the E. coli FtsZ protein could function in several other bacterial species(24). FtsQ oscillation mutant, T0E1, enrichment(11). Its was obtained temperature remedial by 1% sodium chloride (as is several by temperature- sensitivity is salt other fts mutants,94). The ftsQ gene has been subcloned and sequenced(130), from which the DNA sequence predicts that FtsQ protein is 31.4 Kdal in size. However, no radioactive polypeptide has been identified as the FtsQ gene product even though several in vivo labelling procedures were tried that included maxicell and minicells containing ftsQ encoding plasmids phage. and UV irradiated cells infected with ftsQ transducing Thus, FtsQ protein is likely to be either synthesized in very small amount or is extremely labile or both. Filamentous cells of ftsZ and ftsQ mutant at the nonpermissive temperature have similar phenotypes. Both mutant filaments are resistance to 0-lactam lysis and both quickly have septa temperature, 30°C (113). constrictions However, filaments of ftsQ mutant can have indicating initiation step. after a shift back to the permissive that septation can continue pass the Thus the FtsQ gene functions after FtsZ gene but before FtsA, Ftsl genes. Directly blocking DNA synthesis( e.g. dnaA(ts), dnaC(ts), dnaB(ts) etc.) causes inhibition of septation and produces transient filamentous cells(32). pathway: This SOS independent division inhibition(TER 18,37,61,62,115,117) can result in short uninucleoid filaments that can produce normal size cells from their end which are DNA-less. This anucleated cell production requires a functional 9 cAMP-CRP complex and is affected by suIB mutations which suggests an involvement of FtsZ in the TER pathway. of involves the cAMP-CRP division) by CRP, since may The division promoting role activation of FtsZ(that promotes protein(s) or other metabolic factors(67) by the cAMPFtsZ expression is not directly regulated independent division by cAMP- CAP(27). The reversal of the SOS inhibition requires a short period of protein synthesis at the end of a round of chromosome replication(66). termination division protein and its FtsA has been proposed as a possible because it synthesis controls appears to late be events(35,118) coordinated with of DNA replication in the TER pathway(27,115,117). The gene product of FtsA has been postulated to be a membrane protein(53) and a structural component of the septum(116). The ftsA gene has been cloned and DNA sequenced(95); and identified as a 50 Kdal polypeptide on SDS-PAGE of extracts of in vivo labelled cells containing ftsk on plasmid or a X transducing phage(76). The expression of FtsA is very low, even when amplified by introduction of a multicopy plasmids encoding ftsA(109,129). Possible protein interaction between FtsA and PBP3(119) suggests an involvement of FtsA in peptidoglycan synthesis specific for septum formation. The properties combination of double with mutants either the harboring ftsQl, the ftsAlO, Ion ftsA\2, mutation or in ftsZSh mutations suggests that FtsQ, FtsA, and FtsZ proteins also posibly interact with each other and perhaps form a molecular complex(29). FtsKpbpb or sep),which is closely linked but separate from ftsQAZ locus, has been cloned and sequenced(87). Its gene product 10 (60 Kdal) is present membrane(103). Ftsl protein,PBP3(see codes Sect.2), transglycosylase 50 for which activities vitro(14,31,60). inhibition of in about a per minor posesses on Mutations copies cell penicillin both cell binding transpeptidase peptidoglycan affecting in the substrates Ftsl(94) or and in selective PBP3 by 3-lactam antibiotics such as cephalexin(58) impair cell septation and produces filaments. PBP3 is the only known protein target for 3-lactam antibiotics that involves cell septation. 3-lactam induced cellular lysis at potential division sites occurs only after the action of FtsZ,Q,A division genes. The sensitivity of fts mutants to 3-lactam lysis plus other morphogenic and physiological studies (113) are also consistent with ordering Ftsl function after evidence from FtsZ,FtsQ,FtsA function. autoradiographic Furthermore, analysis of indirect peptidoglycan synthesis(127) suggests the PBP3 is not involved in the initiation of constriction but rather in its proper completion. Recently, the envA gene located immediately downstream of ftsZ has been sequenced and mutagenesis(7). found to be an essential gene by insertion The phenotype of a single non-temperature sensitive envA mutant(envAl) is filamentous cell chains that have inner membrane separation but not outer membrane-cell wall constriction. The envA defect is probably due to decreased N-acetyl muramyl-L- alanine amidase activity(128). Minb at 26 min on the genetic map seems to inactivate old septal sites (28,114), since minb mutants produces minicells. Rothfield's group(13) has recently cloned the minb locus and that minb locus codes found for several gene products indicating it is 11 probably a multi-gene operon. the They have suggested that the role of minb locus is localization of the septation sites, since under expression of minB locus results in minicell production. Another fts gene cluster maps at 76 min and contains ftsY, ftsE, and ftsX(48). from ftsY. The Gene ftsS is closely linked location of the heat shock to, but separate regulatory rpoHihtpR/hin), immediately downstreamof this ftsYEX cell gene, division operon, suggests a possible function of the ftsE cluster is linking the cell division process to some of the majorglobal regulatory networks of the cell( e.g. the SOS or DNA repair regulon, the heat shock regulon, the stringent level during division(22,88) bacterial ATP-binding response regulon). Changes of Ca++ and homology of the FtsE protein to proteins involved in membrane transport(50) also suggests a role of FtsE in cell cycle-dependent ion transport. Other fts mutants that may block division includes ftsB(48 min) and ftsH(69 min). Ftsb mutant has been shown to be allelic with nrdB(68,lll) which is involved in the synthesis of DNA precursors. Cell division inhibition of this mutant is growth rate dependent suggesting indirect involvement of FtsB in the septation process. .FtsH was previously identified as a gene involved in some initial stage of septum formation due to a slow stop of cell division at 42°C(1). A possible role of FtsH protein is to regulate the synthesis or stability or/both of PBP3(41). This was suggested by a recent finding that there was absence of PBP3 in cell envelopes of an ftsH mutant at 42°C even when overproducing PBP3(ftsI) plasmid. the mutant strain contained an The function of ftsM, which maps near leu, appear to be in SOS-associated repair. The ftsM mutant are 12 affected in Weigle reactivation and its expression appears to be regulation by LexA (38,39). A possible interaction of FtsM with FtsZ protein has been suggested because there was amplification of the ftsZ7\ mutation phenotype when in the ftsMl mutation was present in the mutant strain(12,89). II. Roles of penicillin binding proteins in morphogenetic cycle. Penicillin binding proteins (PBPs), located in the intermembane structures(4,97) represents a within set of the cell proteins envelope of Escherichia responsible for coli, peptidoglycan biosynthesis involved in cell shape, elongation and septation(55). These proteins are the actual enzymes that catalyze the insertion of new material into the peptidoglycan sacculus and are engaged in the control of cell elongation(106) and cell division(14,63). PBP1A and IB are transpeptidases present at 200 molecules per cell(104) which are responsible peptidoglycan(PG) net. for the crosslinking of the Inhibition of the function of PBP1A and IB by mutations or cephaloridine( a 8-lactam antibiotic) results in an inhibition of PG synthesis causing lethal cell lysis. PBP2(pbpA gene product) is involved in the control of elongation of the rod-shaped sacculus and indirectly cell septation. Mecillinam, a 8-lactam antibiotic that specifically binds PBP2, inhibits cell elongation and produces spherical cell (106) and that cannot divide(63). It has been proposed that the lethality of mecillinam bound to PBP2 is primarily the result of this septation inhibition (26). Most mecillinam-resistant mutant cells were 13 mutations In pbpA. and rodk genes. These mutants have a spherical cell phenotype in the absence of mecillinam and they apparently grow by constant septation(27). in cya, crp genes. Other mecillinam resistant mutants were These mutants have a rod shaped cell phenotyp in the absence of mecillinam. The cya and crp mutants formed filaments when DNA synthesis was interrupted, since the functional cAMP-CRP complex is required for the production of anucleated cell (see sec 1). Recent cya expression studies using a cya-lac transcriptional fusion plasmids(120) have revealed that cya(adenyl cyclase gene) is transcribed during cell elongation and its transcription is repressed during cell septation. Interestingly, it was found that increased expression of cya inhibits cell division resulting in filamentous cell growth. These data support a possible role of the cAMP-CRP complex in cell elongation and suggest cAMP-CRP may have a negative function on cell septation. The fact that cya and crp mutants are mecillinam resistant suggest that the cAMP-CRP regulates some aspects of the interactions between PBP2 and the septation apparatus. Furthermore, the inhibition of lateral wall elongation by mecillinam stimulates suggests septation that crp(ts),ftsk cAMP-CRP mutant(19). complex may be This regulating observation the transition between peptidoglycan synthesis of lateral wall elongation and septum formation. PBP3( the gene product of ftsl, pbpb, sep) is a complex protein of 60 Kdal activities with which transglycosylase is formation(14,31,60). involved Septal in and transpeptidase murein peptidoglycan synthesis synthesis enzymatic and is septum inhibited during the period of actual septation by selective inhibition of PBP3 14 or FtsI mutations. Biochemical data(31) has revealed that PBP3 is responsible for viability when there is a under limitation of pentapeptides, nonseptal since PBP3 can transpeptidations. use the Other available biochemical murein tripeptide studies(31) for have demonstrated that at least part of the septal peptidoglycan, which contains increased amounts of cross linked peptidoglycan with a high ratio of tripeptide cross linked subunits, is synthesized by PBP3 using UDP-N-acetylmuramyl tripeptide-substrates as acceptors. PBP4 a 49 carboxypeptidase Kdal polypeptide and DD-endopeptidase transpeptidase activity in vivo. secondary that has transpeptidation been shown activity in to vitro have and PBP4 is believed to be involved in resulting in additional peptidoglycan cross linking, that in a way converts new murein into old murein(55). PBP5(dacA) is a 42 Kdal polypeptide that has DD- carboxypeptidase activity which is high just before septation(8). PBP5 is subunits responsible into substrate tri- for for and PBP3 converting peptidoglycan tetra-peptide subunits, and septal pentapeptide the peptidoglycan preferred synthesis. Overproduction of PBP5 results in spherical cells, which is the same phenotype exibited by PBP2 mutants. A recent model(8,82,85) for the morphogenetic cycle in E. coli invokes a shifting balance in the relative activities of PBP2 and PBP3 that is driven by periodic changes in the activities of D-alanine carboxypeptidase(PBP5). This model does explain overproduced or pentapeptide chains required for the PBP2 is cell into spherical cell mutated. tetrapeptides elongation because phenotype Increased when conversion suppresses PBP2 PBP5 is of crosslinking requires nascent 15 peptidoglycan chains with complete pentapeptides. The location of dacA(PBPS) gene's chromosomal location is near gene locus with rodk, pbpA genes at 15 min on the E. coli genetic map (105,108). The low numbers of PBP3(50 molecules/cell) and PBP2(20 molecules/cell) molecules which are produced at a constant rate in each cell suggest cycle that from the a small balanced number of activities mRNA molecules(104,126) between PBP2 and PBP3 is obtained by the modulation of their enzymatic activities rather than by their f£sZ84(ts) synthesis. mutants Biochemical exhibit a studies(84) low have activity shown of that D-alanine carboxypeptidase at 42°C when the cells are filamentous, suggesting FtsZ may be a modulator. In addition, other data(31,42) point to FtsA and FtsH as possible modulators of PBP3 either regulating the enzymatic activity and/or protein stability. A possibility for protein-protein interaction between PBP3 and FtsA was suggested by the increase resistance of ftsA mutants to the lytic action of Plactam antibiotics and [125I]benzypenicillin by to nonpermissive temperature. the PBP3 finding in ftsA of decreased mutant binding strains at based on decreased binding of PBP3 to 125I-ampicillin in a strain carrying the sulB mutation. PBP3 and RodA(lO) combination of activities mutation in both rodA or of the cell In proteins may also interact because a mutants with normal shape and division. the the Possible interactions of PBP3 with FtsZ have also been implied(31), which was addition, of division pbpB gene can results in These findings indicate that genes and the enzymatic activities of PBPs are highly coordinated and regulated, possibly as part of a multimeric bifunctional complexes for both cell elongation 16 and septum formation. be, in turn, These multimeric bifunctional complexes could modulated by multiple factors linked to global regulatory metabolic circuits at discrete places(possibly potential division sites) within the cell envelope of E. coli. II. Periseptal anulii and potential division sites. Donachie et al.(36) observed that in dnaA(ts) mutant at the nonpermissive temperature(42°C) unreplicated nucleoid at potential division block sites production of resulted in a of cell septation short anucleoid filamentous cells. Normal less cell were produced from these anucleoid and the sized DNA- filaments when the transient chromosomal replication blockage was removed by returning these cells to the permissive temperature. that the cell continues This finding indicated to develope potential division sites of unicellular length in the absence of new chromosomal replication(50). Thus DNA finding occurs replication and cell septation can be uncoupled. This also indicated that the initiation of the septation process before completion of chromosome replication. Jones and Donachie(66) and by Holland(52) have proposed that activation of cell septal division replication. sites occur after the termination of chromosome In fact, Donachie and Beggs(34) found that potential division sites couldbe detected by their sensitivity to penicillin and that these sitesappear to be present at regular intervals early in the cell cycle. Teather et al (114) proposed that the synthesis of quantumly produced division factor frequency of the cell septa. controled the location and This hypothesis was to explain why a 17 normal potential division site was lost in the miriB mutant, in which the synthesis of a minicell results in the parent cell usually being two cell lengths. Recently, Rothfield's group observed these potential division sites by TEM in as serial sections of plasmolized bacterial cells(78) concentric rings of tightly bound inner-outer membrane [commonly called adhesion zones(5)]. "periseptal annuli". They called this organelle the The periseptal annuli flank the constriction site around the potential septa and they appear to act as an innerouter membrane between gasket compartmentalizing developed a membrane isolation of a membrane fraction, called outer membrane lightCOMjJ, Biochemical involved fractionation this studies(59) in the Ishidate procedure inner-murein-outer demonstrated translocation that et space dividing contains 3). periplasmic the which cells(Fig the al.(59) that enables membrane this of have 0ML newly the fraction. fraction is synthesized lipopolysaccharides and peptidoglycan synthesis. Additional evidence, supporting the model that the periseptal annuli is an cellular organelle associated with cell septation, is that the 0ML fraction was increased in cell division mutants, IkyD and cha. Both mutants are defective formation(21). in outer Incomplete, membrane nascent invagination during septum annuli do extend that not completely around the cell were also observed at nonseptal regions along growing celIs(25). These nascent annuli appear to be generated from complete annuli already in position at the midpoint of newborn cells, and were displaced laterally during cell elongation to position at 1/4 and 3/4 cell lengths that become the midpoint annuli 18 of newborn cells of forthcoming divisions(25). These newly generated annuli have been hypothesized to be the cell structure involved with chromosome partitioning during the cell division. The finding of periseptal annuli at potential division sites in a divC mutant filament(26), in which cell division was blocked before any septal invagination, indicated that divC gene product acts after biogenesis, maturation and localization of the periseptal annuli. Thus,the formation of the periseptal annuli precedes the initiation of septal invagination. The identification of a second division related structure, septal attachment sites, SAS(79), suggest that it may be a first step in septal invagination. The miriB locus has been proposed(13) to be involved in the localization of the division septum. The single polar periseptal annulus, which remains at the cell pole in each daughter from the preceding division event, could be the foci for the abnormal polar division and minicell formation in miriB mutant strains(13,29). IV. Preliminary studies on the regulation of ftsQAZ gene expression. The 2 min region of E, coli genetic map including ftsQAZ genes contains a large cluster of 14 genes, approximately 20 kilobases, involved in cell envelope growth and division (96,Fig.l). The close clustering of these genes suggest a possible coordinate expression and/or interaction between the genes. The cloning of DNA from this region and the sequencing and subcloning of DNA fragments into promoter assaying have enabled the construction of a transcription map of the region(95,96,109,129,130). Although the contiguous genes 19 between dd!(D-ala:D-ala ligase) and envA are transcribed in the same direction, this regions forms an atypical operon with overlapping transcriptional units since each gene, when expressed from its own promoter(110,130). subcloned, can be The promoters (Pz3 and P«) of the ftsZ gene are within the coding sequence of the upstream ftsA gene(110), the promoter of ftsk in turn is within the coding sequence of upstream ftsQ gene(95), and the promoter for ftsQ may be within its upstream gene neighbor. SI mapping (Corton and Lutkenhaus, unpublished) and the used of a terminator-probe vector has recently independent revealed the location of a typical strong rho- transcriptional terminator just downstream of envA(7). The absence of strong transcriptional terminators between ddl and envA suggests that transcription originating from ddl could continue through to transcripts envA. are However,it produced different metabolic signal form remains this gene to be cluster determined in what response to in vivo. A possibility is that gene expression involves promoter specific transcription initiation and RNA polymerase transcriptional pausing. A computer search of the published DNA sequence of the ftsQ-ftsA region(95) has identified two potential NusA sites with a possible DNA sequence that could form a stem-loop structured!. Gayda, unpublished data), one at the beginning of the ftsQ gene sequence and the other near the ftsZ promoter Pz3. NusA specific DNA sequence has been demonstrated to be necessary for lambda N gene antitermination(43). The expression study of ftsZ gene has revealed substantial reduction of ftsZ synthesis when a TnlOOO transposon was inserted within the ftsA gene(64). Similarly, an ftsZ encoding DNA fragment, 20 containing only the two promoter (Pz2 and PZi) cloned into X vector, was insufficiently expressed to complement an ftsZ mutant. However, a X ftsZ transducing phage was able to complement a ftsZ mutant(74) when it contained additional upstream DNA including the promoter(Pz3) within the ftsA gene but not the entire Sullivan and Donachie(109) found and Pz3 ftsA gene coding region. that the transcription from Pz2Pzi when togetherwas more than the sum of their separate promoter activities. Similarly,there is evidence(33) that upstream DNA the ftsA in addition to promoter is required for proper expression of the ftsA gene. These data suggest that regulation of the ftsQAZ genes is not simple but complex. Transcriptional expression of ftsQAZ encoding region also seem to involve promoters. different regulatory interactions amoung the various The amount of FtsZ per cell was invariable over a 10 fold range of cell mass, thus suggesting that ftsZ expression is subject to some type of autoregulation(36,65). found to expression be increased in a may be negatively Transcription from Pz3 was cya mutant, regulated which by suggested cAMP-CRP ftsZ complex. However,(as previously mentioned) a recent study(27) has demonstrated that cAMP-CRP does not regulate ftsZ gene expression but that the increased small cell FtsZ protein inthe cya mutant size of the cya mutant. was a compensation for the Transcription from Pz3 was increased as much as 10 fold when ftsA had a nonsense mutation and to a lesser extent in mutants of ftsl, ftsQ and ftsZ, Post-transcriptional processing and/or modification of the Fts gene products have not been reported. Materials and Methods I. Bacterial strains, plasmids and phages. The E. coli K-12 strains, plasmids and phages used in this study are listed in Table 1 and 2. 100 yl of A1098(10 X 10® plaques forming units) that contains a mini-TnlO transposon were mixed with 100 yl of an overnight culture of strain RGC103-9(sulB9,Ion). After 20 min at room temperature the mixture was plated out on a YET-plate containing tetracycline(12.5 yg/ml). YC99 was Tetracycline-resistant (TetR ) colonies were pooled. constructed by Pl-mediate transduction of Strain RGC123 to tetracycline resistance and nitrofurantoin (NF) resistance (to select for su2B9 allele) from such a random TnlO bacterial pool. SulB9 allele was found to be 90% cotransduced with TnlO in YC99. YC100 was constructed by PI mediated transduction of ftsAlO allele linked to TnlO (ftsA:Tnl0) into strain AMC290 by selecting resistance and temperature sensitivity. for tetracycline Strain YC200 was obtained by Pl-mediated transduction of Lac+ allele from Hfr3000 into AMC290 by isolating blue colonies on minimal lactose plates containing X-gal(40 yg/ml) after 3 days incubation at 30°C. II. Media and growth conditions. E. coli K-12 strains were routinely grown on complex medium, YET (per liter): lOg Bacto-tryptone(Difco), 5g Bacto-yeast extract(Difco) and lOg NaCl. The final pH was 7.0. YET agar plates included 15 g/1 Bacto-agar(Difco). Low salt complex medium, TEY was identical to YET 21 22 but without any NaCl. Other media used for E. coli growth included: (i) M9 minimal medium(83) which consisted of minimal salts (per liter): 6g Na2HPOA, 3g KH2P0A, 0.15g NaCl, and lg NHACl,to which after autoclaving and cooling were carbon source was 0.2% glucose added plus lOg glucose uridine per liter. and and O.lmM CaCl2,the and amino acids and vitamins added as required. The final pH was 7.4. salts 2mM MgSO* (ii) KU-medium(lOO) was M9 minimal lOg NZamine(Sheffield products), 0.5g (iii) Sulfate free Hershey medium(lOO) that was (per liter) 4g glucose, 1.0 mg thiamine(B1), 5.4 g NaCl, 3g KC1, l.lg NH*C1, 15 mg CaCl2-2H20, 203 mg MgCl2-6H20, 0.2 mg FeCl3-6H20, 87mg KH2POa, 12.lg Trizma base(tris[hydroxymethyl]aminomethane). The final pH was 7.0. (iv) Lactose agar plates(83) consisted of (per liter) 10.5 g K2HPOa, 4.5 g KH2POA, 0.5g Bacto-agar, (NHA)2S0A, 0.5 g sodium citrate, to which after autoclaving, 15g 1.0 ml of 20% MgS0A-7H20, 0.2ml of 5% thiamine, 10 ml of 20% lactose, 5ml of 2% amino acids as required were added. Final pH was 7.0 without adjustment.(v). L medium consisted of(per liter) 10 g Bacto-tryptone, 5 g Bacto yeast extract and 5 g NaCl. Carbenicillin kanamycin(kan,25 (carb,25 yg/ml, yg/ml), and tetracycline(tet,12.5 yg/ml) 5-bromo-4-chloro-3-indoly-(5-D- galactopyranaside(X-gal, 40 yg/ml) were added when needed. Overnight cultures at 30°C were diluted approximately 100 fold in fresh media and grown to 0D6Oo = 0.1 to 0.5 before cells were challenged by experimental conditions such as a temperature shifts. The permissive temperature for the ftsZ, /tsA, ftsQ, ftsl mutants was 30°C and the restrictive temperature was 42°C. 23 ftsk mutants, YC100, was unable to grow on TEY plates at 30°C, and exhibited temperature sensitive phenotype on YET media at 42°C. ftsl mutant,AMC493, was sensitive to growth at 42°C on both YET and TEY media. ftsZ mutant,AMC419, and ftsQ mutant,T0E1, was temperature sensitive only on TEY (no salt) media. Growth of ftsZ, ftsQ mutants on YET plates incubated at 42°C results in colonies. Strain YC200( laC") was induced for expression of 0-galactosidase with ImM IPTG(isopropyl 0-D-thio-galactopyranoside) at 0D6Oo = 0.12 for 2 hrs. Ill Enzymes and chemicals. Restriction endonucleases were obtained from either New England Biolabs,Inc., or Bethesda Research Laboratories(BRL). T4 DNA ligase, bacterial alkaline phosphatase and the large fragment of DNA poll (Klenow) were obtained from BRL. p-chloromercurophenyl sulfonic acid(pCMS), used as an inhibitor of contaminating nuclease activity, DL-dithiothreitol, and Trizma Base (tris[hydroxymethy] aminomethane) were purchased from Sigma Chemical Co. Alkaline-phosphatase linked goat anti-rabbit imunoglobulin G antibody (Western blot AP system, W3930) was obtained from Promega. The goat anti-rabbit-immunoglobulin G antibody labelled with lOnm gold particles was purchased from Jenssen (Pharmaceutical). 0-lactamase substrate, PADAC([l-(thienyl-2-acetamido)]-3-[2-(4- N,N-dimethylaminophenylazo)pyridium methyl]-3-cepem-4-carboxylic acid) was purchased from Calbiochem(Behring Diagnostics). 24 IV. Methods of DNA preparation a. Large scale plasmid DNA isolation. Plasmid DNA was prepared by the method of Robert Kolter with some modifications. Five mis of an overnight culture of cells that were grown in L broth plus 40 pg/ml ampicillin were used to inoculate 1 liter fresh L+amp. The culture was grown at 37°C (or 30°C) with shaking(200 rpm) to a optical density of 0D6Oo=0.4. amplified continuing in the cells incubation by adding overnight with Plasmid DNA was chloramphenicol(150mg/l) shaking. The cells and were harvested by centrifugation for 5 minutes at 5,000 X g which yields a soft pellet of cells. These cells were then resuspended in 5 ml of 50mM Tris-Cl(pH 8.0) with 25% (w/v) sucrose. 0.5 mis of lysozyme (lOmg/ml in a solution of 50mM Tris-Cl,pH 8.0, and 25% sucrose) was added and the mixture was incubated on ice for 5 minutes. Then, 0.5ml of 0.25M EDTA (pH 8.0) was added and further incubated on ice for another 5 minutes. Next, 5 ml of Triton X-100 lytic mixture (50mM Tris-Cl(pH 8.0),62mM EDTA and 0.4% Triton X-100) at room temperature was added quickly, mixed, and left at room temperature for 5 minutes or until a clear lysate was achieved. Cellular debris was sedimented by ultracentifugation at 40k r.p.m. for 45 minutes in a Beckman type 75 Ti rotor. centrifuge tube. The supernatant fluid was decanted into a plastic One gram of CsCl and 0.1ml of a solution of 5mg/ml ethidium bromide was added per ml of supernatant. The protein debris was then removed by centrifugation at 10K rpm (Sorvall SS34 rotor) for 5 minutes. The supernatant was transferred to quick-seal polyallomer tubes and CsCl density gradients were established by centrifugation to equilibrium at 49k rpm in a Beckman VTi65 rotor for 20 hrs at 15°C. 25 Covalently closed circular plasmid DNA band was visualized with long wave ultraviolet light and extracted from the side of the tube with a number 21 needle and syringe. To remove the ethidium bromide, plasmid DNA in CsCl saturated butanol. solution was extracted 6 times the with water- The solution was then diluted with two volumes of water; the DNA was precipitated by the addition of ethanol at 6 times the original volume, and solution kept at -20°C for 2 hours. The plasmid DNA was pelleted by centrifugation at 10,000 X g, washed with 80% ethanol, dried in vacuo, and resuspended in T.E.(10 mM Tris-HCl, ImM EDTA, pH 8.0) buffer. b. Small-scale (rapid) plasmid isolation. Small quantities of plasmid DNA were prepared for rapid screening by the method of Holmes and Quigley(54) with modifications. 5 mis of bacteria that were grown overnight were pelleted at 3000 X g for 5 min and resuspended in 0.35 ml of STET2 buffer( 8% sucrose, 0.05% Triton X-100, resuspended cells 50mM 25 yl EDTA, of lOmM Tris-HCl(pH freshly prepared 8.0)). To the lysozyme(lOmg/ml) was added, mixed briefly and then the tube was placed in a boiling water bath for 40 secs. The tubes were chilled in ice bath and immediately centrifuged at 12,000g for 20 min at 4°C. The supernatant was drawn, 1/10 vol of 3M sodium acetate (pH 4.8) was added and then an equal volume of cold isopropanol was added and the solution was placed at 18°C for 10 min. The DNA was precipitate was pelleted by centrifugation in a microcentrifuge for 5 min at 12,000 g and washed 2 times with ether. The resulting plasmid DNA pellet was resuspended in 50 ul of TE buffer and kept at -18°C. 26 V. General genetic and recombinant DNA techniques. PI transduction previously for described strain construction (80,102). Similarly, were A performed as growth and phage infection were as described (80,102). DNA recombinant techniques described by Maniatis et al (81). were performed essentially as Restriction enzyme digestions were for 60 min at 37°C in buffers recommended by the manufacturers. When DNA was to be digested with more than one enzyme, the enzyme reaction requiring lower salt concentration was performed first, then reaction mix was inactivated by heating at 65°C for 10 min, cooled and adjusted by the addition of salt to meet the requirement of the next enzyme in the sequence. 10 mM to After completion of enzyme digestion, pCMS was added to inhibit nuclease activity (without interfering with subsequent ligation reaction) before the usual inactivation at 65°C for 10 min. Otherwise, the digestion reactions were terminated by phenol extraction of proteins followed by ethanol precipitation of the DNA. In some restriction DNA enzyme fragment sub-clonings, digestion was the vector dephosphorylated DNA with after bacterial alkaline phosphatase(0.1 unit/yg of DNA) at 65°C for 1 hr. The DNA was then ethanol precipitated after phenol-chloroform ether extraction, and then resuspended in TE buffer( lOmM Tris-HCl, ImM EDTA, pH 8.0). DNA fragments elution onto from agarose dialysis membrane gels as were described recovered in by section electro VII, and purified through either Elutip-D columns according to manufacturer's specification or by chromatography on a DEAE-50 column. The DNA 27 fragments were further cleaned by pheno-chloroform-ether extraction and then ethanol precipitation. DNA pellet was finally resuspended in TE(10:1) buffer. Ligation of DNA fragments were performed in ligation buffer ( 66mM Tris-Cl, pH 8.0, ImM EDTA, 5mM MgCl2, 5mM dithiothreitol, 0.1M ATP). Optimal ratio of vector DNA to insert DNA was determined as described by Goodman et al (40). Plasmid DNA described(70). transformations were performed as previously Unless otherwise noted, strain AMC290 was used as the recipient in the DNA transformations. The orientation and in some cases whether or not a vector has an insert DNA fragment was determined by restriction enzyme cleavage mapping of isolated plasmid DNA as previously described(107). VI. Bacterial transformations. Transformation Lederberg and with Cohen(70) plasmid as DNA followed previously a modification described.(101). cells of appropriate host were prepared as follows. of Competent A 50 ml L broth culture was inoculated with 0.5 ml of an overnight culture and grown to 0 D 6Oo = 0.2. resuspended in The cells were chilled on ice, 50 mis of cold 0.2M MgCl2. centrifuged and After a second centrifugation at 4°C, the cell pellet was resuspended in 25 ml of 0.1M CaCl2 and chilled on ice for 20 minutes. in the cold was carried out, and the cell resuspended in 0.7 mis of cold 0.1 M CaCl2. mixing 0.1ml DNA( approximately ,05yg up A third centrifugation to pellet was finally Transformation involved lyg) with 0.2 competent cells and incubating mixture on ice for 30 minutes. ml of To aid 28 DNA uptake the mixture was heat pulsed 30 secs before 30 minute at 37°C ice incubation transformation and mixture after was for diluted 2.5 2 to minutes at 10 with fold 42°C. L The broth containing 10% glycerol and incubated at either 37°C for lhr or 30°C for 2 hrs before spreading onto selective agar plates. were verified by antibiotic resistance and/or Transformants phenotype( i.e temperature resistance) . After intial transformation with a newly constructed plasmid, the plasmid DNA was isolated and purified as described in section IV.b and then used in a second transformation to determine the phenotype of the new plasmid in the appropriate strains. VII Electrophoretic techniques. Horizontal buffer agarose gels were prepared in TBE electrophoresis (89mM Tris-borate,2 mM EDTA, pH 8.0). Loading buffer(6X) contained 0.25% bromphenol blue, 0.25% xylene cyanol and 15% Ficoll in dH20. After electrophoresis, the agarose gels were stained with a solution of ethidium bromide(0.5 mg/ml) for 1 hr. on a U.V. trans-illuminator. DNA was visualized Hindlll digested lambda DNA was used as DNA fragment molecular weight markers. Sodium dodecylsulfate(SDS) polyacrylamide gel electrophoresis (PAGE) was performed utilizing the discontinuous system as described by Laemmli(69), that was modified as described previously(44). The main gel was polymerized in a solution containing 0.37M Tris-HCl(pH 7.8), 0.1% SDS, 0.03%(vol/vol) TEMED, 0.33%(wt/vol) ammonium persulfate, with concentration of acrylamide ranging from 10 to 30% maintaining a N,N'-methylene bisacrylamide-acrylamide ration of 1:173. 29 10% to 30% linear gradient acrylamide gels were prepared with a gradient maker (Hoefer Scientific Instruments). The stacker consisted of 0.14M Tris-HCl(pH 0.15% N,N'-methylene 6.8), 5.7 % acrylamide, bisacrylamide, 0;1% SDS, 0 .05%(vol/vol) TEMED, 0.05%(wt/vol) ammonium persulfate. The electrode buffer at pH 8.5 contained 0.05 M Tris-HCl, 0.384M glycine and 0.01% SDS. The gels were run at 20 milliamps at constant current until the dye front reached the gel bottom. Protein extracts were solubilized by heating at 100°C for 5 min (at 65°C for 30 min for certain membrane fractions) in the presence of 1% SDS, 0.625 Tris-HCl(pH 7.0), 5% 8-mercaptoethanol, 4M urea, bromophenol blue. Protein in SDS-PAGE and 0.015% were stained and fixed with a solution of 0.25% Coomassie Brilliant Blue, 43% methanol and 7% acetic acid and destained in 43% methanol and 7% acetic acid. VIII. Extraction of DNA fragments from agarose gels. DNA fragments were extracted from agarose gels by just ahead of the desired band, placing a cutting aslot piece of dialysis membrane to line the distal side of the slot, filling the slot with IX TBE and then electro-eluting the DNA into the slot and against the dialysis membrane. The DNA was pulled off the membrane by reversing the current for 30 seconds, and the buffer in the slot was collected. The slot was rinsed two times with buffer which was also collected. The DNA fragments was cleaned by passage through an Elutip-D column(Schleicher and Schuell) or by DEAE-52 chromatography. DNA from column was concentrated by the addition of one-tenth volume 3M sodium acetate(pH 4.8) and ethanol precipitation. 30 IX. Maxicell labelling of plasmid specified proteins. The procedure used was a modification of Sancar et al. (99,100). CSR603 was transformed with specific plasmid DNA to be analyzed for protein expression. Three mis KU medium inoculum of a plasmid containing maxicell strain was grown at 37°C to an 0D&oo of 0.2 (2 X 10s cells/ml). These cells were then diluted 1:50 into fresh KU medium and grown again to an 0D6Oo of 0.2. then transferred to a 6 cm petri dish (Falcon). 10 ml of The cells were The dish containing the cells and a small magnetic stirring bar was placed onto a magnetic stir plate. A portable 254 nm ultraviolet light(ultraviolet products, Inc.) was clamped to a stand at a distance of 20 cm from the dish. The top of the petri dish was removed and the vigorously stirred cells were irradiated for 30 seconds. The cells were then transferred to a 125 ml flask, diluted 1:2 with K medium and incubated for 2 hours at 37°C. It should be noted that all incubation were carried out in the dark as the bacteria are photo-reactive. Cycloserine(200 yg/ml) was added to each cell suspension and the incubation with shaking was continued for 13 to 14 hours.An addition 200 yg/ml cycloserinewas added during the final hour incubation. centrifugation at 6000 rpm(SS34 The cells were sedimented by rotor) at 4°C. The supernatant was discarded and the cells were washed twice with sulfate-free Hershey medium(50 ml flask) and incubated with shaking for 1 hour at 37°C. The plasmid specific protein were labeled for lhr at 37°C with [35S]- methionine(New England Nuclear) which was added at a concentration of 5 yci/ml. 6000 The labelled maxicells were pelleted by centrifugation at rpm and washed radioactivity. All twice with 0.1M NaCl supernatants and to washes remove were non-specific treated as 31 radioactive waste. The cell pellets were then resuspended in 200 yl of sample buffer and boiled for 5 minutes to denature the proteins. The cpm/yl of samples Scintillation counter. loaded onto 10% were determined in a Beckman LS6800 Approximately 500,000 to 750,000 cpm/lane was to 30% gradient SDS-Polyacrylamide gel and electrophoresed at 20 mA constant current until the dye front ran off the end of the gel(approximately 16 hours). The gel was then dried at 80°C on a BioRad gel dryer. Dried gels were placed directly against Cronex X-ray film(Dupont). Development and visualization of the resulting autoradiograms were as previously described (44,101). X. Plasmid constructions. a. Ia vivo mini-Mu transposon mutagenesis for the construction of FtsA-LacZ fusions. The procedure developed transposon mutagenesis mutation of of plasmid MudII1734(Fig. by plasmids pRGC31. strain Casadaban(20) was used The to mini-Mu P0II1734(20) which for isolate mini-Mu insertion transposon 1) is a 9.7 Kb derivation of Mudll confers kanamycin resistance(kanr). into M. phage(20) used, that Plasmid pRGC31 was transformed contained a Mucts and mini-Mu, MudII1734. The strain was heat induced and the resulting phage lysate was used to infect strain YC99. Phage transductants that conferred both the original plasmid resistance(ampicillin from pRGC31) and miniMuresistance to Phage kanamycin contained plasmids transductants that expressed with mini-Mu inserts. P-galactosidase activity, indicating a gene fusion with LacZ, were identified with X-gal on 32 selective plates(YET orientation of with kan the mini-Mu and amp, 101). The location inserts were determined by and restriction enzyme mapping of the isolated plasmid DNA( Fig 6). b. Construction of FtsA-LacZ overproducing plasmid, pYC3. Plasmid pGW7(30) is derived from the E. coli plasmid pBR322 and contains a 4.0 Kb EcoRl-BamHl fragment derived from the bacteriophage lambda control region (Fig. 4). genes to be placed under promoter. The PL the control promoter is of regulated This enable foreign the highly active by the repressor protein (CIasr) contained on the plasmid. A PL thermolabile Cl Also present is the A N gene whose product allows for efficient readthrough of rhodependent and rho-independent terminator site. The plasmid pGW7 and described in section IV. a. pYC16 were isolated and prepared as The plasmid vector pGW7 was completely digested with BamHi and followed by treatment with bacterial alkaline phosphatase. The plasmid pYC16 was partially digested with BartSW (lu/4yg, 3 min at 37°C). The 8.5 Kb BamHl DNA fragment containing the FtsA-LacZ hybrid gene was recovered from an agarose gene and purified as described in section VII. The two DNA fragments were together and then transformed into strain AMC290( Lac). ligated Transformants that grew as blue colonies on X-gal,amp, kan plates were selected. The insertion and orientation of the 8.5 Kb DNA fragment was determined by restriction enzyme mapping of one isolated plasmid DNA, which was designated pYC3. 33 c. Construction of promoter fusion plasmids. The ftsZ regulatory region the appropriate purified subcloning promoter probe plasmid, gene fusions were restriction performed fragment into by the pKK232-8(16,Fig. 5) that give transcription fusions to the chloramphenicol acetylase gene (CAT). pRGC31 was used as the source of ftsZ DNA. pYC500 consists of a agarose gel purified 1.7Kb BanMl-Hindlll restriction fragment from pRGC31 that was ligated into BamHl, Hindlll digested pKK232-8. Carta*-, Cmjr(25ug/ml) transformants were verified by restriction enzyme analysis of BamHl - Hindlll digestions and Bglll digestions. In pYC530 the BamHl-Bglll DNA fragment of pYC500 was deleted by a double digestion and ligation. Again, restriction analysis of carb*, cm* transformants verified the construction. pYC580 consists of an agarose gel purified 0.5Kb Hindlll-EcoRl restriction fragment from pRGC31 in which the overlapping DNA ends were filled in with DNA polymerase I and then blunt end ligated into Smal ends of pKK232-8 that were dephosphorylated. One carb*, cm* plasmid transformant contained the proper DNA fragment in the desired orientation based on Hindll and BssHII double digestions. pYC600 consists of an agarose gel purified 2.2 Kb BamHl-BcoRl restriction fragment from pRGC31 in which the overlapping DNA ends were filled in with DNA polymerase I and then blunt end ligated into the Smal ends of pKK232-8 that were dephosphorylated. Seven carb*, cm* the transformants contained the proper fragment in desired orientation based on Hindll-Bglll double digests and Hindll digest of plasmid DNA. 34 pYC620 consists of an agarose gel purified 1.2 Kb BgHI-fcoRl restriction DNA fragment form pRGC31 in which the overlapping DNA ends was filled in with DNA polymerase I and then blunt end ligated into the Smal ends of pKK232-8 that was dephosphorylated. Plasmid transformants were verified to contain the proper DNA fragment based on Windlll digestions. XI. Purification of FtsA-LacZ hybrid protein. The purification galactosidase of affinity FtsA-LacZ columns as fusion protein described by was by Bastia(47) Pand Ullmann(121) with some modifications. E. coli cells AMC290/pYC3 were grown in YET broth at 30°C in the presence and carb(25 yg/ml). The X pL promoter of pYC3 was induced by heating to 43°C when the culture reach an 0DfeOo of 0.3. After 2.5 hrs at 43°C the cells were harvested by centrifugation at 6000 g for 5 min. Pellet was suspended so that a gram of cells was in 2 mis of buffer I (0.2M Tris-HCl, pH 7.0/ 0.25M NaCl/O.OlM magnesium acetate/ 10 mM 2-mercaptoethanol/ 5% glycerol/ ImM PMSF). was frozen at -80°C and thawed quickly in This cell suspension a 30°C water Pancreatic DNase and RNase was added at 10 yg/ml each. bath. The cell suspension was then disrupted by two passages at 10,000 psi through a pre-chilled French pressure cell. Unbroken cells were centrifugation for 10 min. at 2000 X g. centrifugation at rotor(Beckman). 35,000 removed by The lysate was clarified by rpm for 30 min at 4°C in a type 75 Ti The P-galactosidase activity in the cleared lysate was determined as described(83). 35 The protein ammonium sulfate. stirring to 40% from above cleared lysate was salted out with Ammonium sulfate crystals were added slowly with saturation. The precipitate was collected by centrifugation and the pellet was suspended in buffer II (lOmM Tris, pH 7.0/ lOmM magnesium acetate/ 0.2M NaCl/ 0.1M KC1/ O.lmM EDTA/ ImM DTE/ O.lmM PMSF) and dialysed against 100 vol of the same buffer for 3 hrs with 3 buffer changes. The above dialysate was mixed with 5M NaCl to obtain a final concentration of 1.5M NaCl. A volume of 2 ml of this solution was applied to 2 ml bed volume of galactopyranoside-agarose(NH2BenSGal-Agarose) p-aminobenzyl-8-D-thiocolumn which was equilibrated with buffer III (lOmM Tris, pH7.0/ 1.5 M NaCl/ 0.1M KC1/ 0.1M EDTA/ ImM DTE/ O.lmM PMSF). The flow rate was 1 ml/hr. The column was washed with buffer III (about 100 bed volumes) until no more material absorbing at 280nm appeared in the flow through. The protein retained was eluted from the column with 0.1M sodium borate, lOmM 3-mercaptoethanol, pH 10, precipitated with ammonium sulfate. and the proteins was promptly This precipitate was collected by centrifugation and the protein was resuspended in buffer IV (40mM Tris-HCl,pH 7.5/ImM MgCl2/0.ImM EDTA/ImM DTE/50% glycerol) and kept at -80°C. Before injection into rabbits the protein was dialysed against phosphate buffered saline(PBS; lOmM phosphate buffer/150mM NaCl, pH 7.4). XII. Production of FtsZ-LacZ Antibodies Antiserum against the FtsA-LacZ fusion protein using a immunization protocol previously described(98). was produced A rabbit was 36 injected intradermally with antigen(500 yg) emulsified in an equal volume of complete Freund adjuvant(Difco). After 4 week, the rabbit was given a booster injection of another 500 yg of antigen in same volume with incomplete Freund adjuvant. To insure high titer of FtsA specific antibodies, the rabbit was given a second booster injection with 200 yg of FtsA-LacZ hybrid protein isolated from SDS-PAGE gels. Strips of acrylamide excised from gels which had been stained and dried were rehydrated in PBS buffer and macerated in a mortar and emulsified in an equal volume of incomplete adjuvant. Blood was obtained before experiments (pre-immune serum). was titered at 1 week the initial injection for control Antibody production against FtsA-LacZ intervals after the booster injection. Detection and titer of the blood antiserum were carried out by ring precipitin and dot blotting tests (17) with aconstant dilution of antigen. Pure immunoglobulin,IgG antibody fraction, was prepared from the serum by chromatography on protein according to manufactures procedure. A-sepharose C1-4B column Affinity purified rabbit IgG was dialysed against PBS buffer containing0.02% sodium azide overnight with four changes immunodetection, of buffer. Toenhance the specificity of the IgG antibodies were absorbed against a cleared lysate of YC100 (ftsAlO mutant) prepared after a temperature shifted to 42°C for 2 hrs. XIII. Western blot analysis. Polypeptides separated on a 10 to 30% gradient SDS-PAGE gel were electrophoretically transferred to a Nytran Sheet (Schleicher & 37 Schuell) at 250 mA for 3 hrs by the method of Burnette(17) with the following modifications. The transfer buffer consisted of 20 mM Tris-Cl, 150 mM Glycine, pH 8.0. Transfer standards (BRL). buffer was monitored by prestained molecular weight The immobilized polypeptides were blocked in TBST (0.1M Tris-Cl,pH 7.5, 0.1M NaCl, 0.05% v/v Triton X-100) containing 10 % (w/v) bovine serum albumin(BSA) overnight at 48°C. The blocked membrane filter was then incubated with appropriate dilutions of antibodies (1:1000) in TBST buffer containing 0.5% BSA for 2 to 4 hrs. each), After being washed in six changes of buffer (10 min the membrane sheets were phosphatase incubated for linked-goat anti-rabbit IgG 1 hr with alkaline (1:7500 dilution) and then washed as described above. All the above steps were performed with gentle agitation at room temperature throughout unless otherwise noted. Antibody reactivity was detected with color development by the substrates BCIP and NBT. If labelled proteins were used, the resulting immunoblot was subjected to autoradiography after being dried. XIV. Cell fractionation procedure. Membrane, cytosol and Sarkosyl insoluble membrane fractions were prepared as described previously (44,101). Ten mis of cells or labelled maxicells with plasmids were pelleted by centrifugation at 12,000 X g for 10 min, suspended in 5 ml of 10 mM phosphate buffer, pH 7.4, centrifuged again, and finally resuspended in 2 mis of the same buffer. The cells were then lysed by sonic oscillation. The un-lysed cells were removed by centrifugation at 2000 X g for 10 min. This 38 cleared lysate was fractionated into cytosol and membrane fraction by centrifugation at 20,000 X g for 15 min. The envelope-containing pellet was then suspended in 2 mis of buffer. envelope pellet preparation was further fractionated into Sarkosyl insoluble outer membrane by being 37°C for 20 min. One half(lml) of the solubilized with 1% Sarkosyl at The total membrane and Sarkosyl-insoluble membrane preparations were centrifuged again at 20,000 X g for 15 min and resuspended in phosphate buffer. were concentrated by precipitation The soluble cytoplasmic proteins with 10% trichloroacetic acid, washed twice with 80% cold acetone and resuspended in smaller volume of phosphate buffer. Proteins from the above fractions were separated by PAGE-SDS gels, and in case of labelled proteins from maxicells were detected by autoradiography. XV. Isolation of membrane fractions 0MH , 0ML , IM. Cell fractionation of membrane envelope into 0M„, OMt,, IM was performed by the procedure of Ishidate et al (59) as briefly described below. Cells were grown at 30°C in YET medium (1 1) to ODeoo31 0.4, rapidly cooled and then centrifuged at 2000 X g for 10 min. Pellet was resuspended in 10 mis of 20% sucrose and (10 yg/ml each) were added. pancreatic DNase and RNase The cell suspension was then mechanically disrupted by two passages at 1000 psi through a pre-chilled French pressure cell. (American Instrument Co., Silver Spring, MD.). After removal of unbroken cells by centrifugation for 30 min at 2000 X g, EDTA was added to the supernatant fraction to a final concentration of 39 5 mM. The supernatant (1.7 ml) was layered onto a two step gradient(SGO) consisting of 0.8 ml of 60% sucrose and 2.5m of 25% sucrose. The crudemembrane fraction, isolated from SGO by centrifugation in a Beckman SW50.1 rotor at 40K rpm for 3.5 hrs, was diluted with lOmM Hepes, 1.365-1.37 at 20°C. applied to the (0.5ml), 55% pH7.4, The top of (0.8ml), 5mM EDTA to a refractive index of resulting suspension (2.0 ml) was the following sucrose gradient (SGI) 50% (2.2ml), 45% (2.2ml), 40% then : 60% (2.2ml), 35% (1.4ml), 30% (1,0ml). The gradient was centrifuged in a Beckman SW41 rotor at 36K rpm for 16 hrs. Fractions (0.3 to 0.4ml) were collected from the top using a fraction recovery system (Beckman) and refractive index was determined with a refractometer (Bausch & Lomb). the refractive concentrated by index, each membrane fraction Based on was pooled and centrifugation at 50K rpm for 3.5 hrs after being diluted with 3 volumes of 10 mM HEPES buffer(pH 7.4), and the final pellets were resuspended in lOmM HEPES buffer. Total cytosol centrifugation of and the membrane supernatant fractions (3 ml) were prepared obtained above by by centrifugation at 100,000 X g for 1.5 hr after dilution with 2 volumes of 10 mM HEPES, pH 7.4. Pelleted membrane was resuspended in same buffer and soluble proteins were concentrated by precipitation with 10% cold trichloracetic acid(TCA). The TCA precipitate was allowed to stand on ice for 20 min, pelleted by centrifugation, washed twice with 80% acetone, and finally resuspended in same buffer. Approximately equal amounts of protein from each sample (50 pg) were loaded onto SDS-PAGE gels used for protein separation and Western immunoblotting as described above. 40 XVI Immunogold labelling and electron microscopy. Immunogold labelling was performed as described by Bayer et al.(6) and by Reid et al.(93) with following modifications. Cells ( AMC290) were grown in YET broth at 30°C to mid-log phase and a portion of cells were then fixed with an equal volume of L broth with 4% formaldehyde and 0,2 M cacodylate-HCl buffer(CD), pH 7.2 for 30 min. The fixed cells were collected on a filter by drawing about 2 mis of the suspension of fixed cells into a 5 ml syringe and passing through an attachable filter holder containing a 13 mm diameter polycarbonate membrane filter (0.40 urn). The filter was washed with 3 ml of 0.1M CD buffer and then dehydrated with graded alcohol series(50% to 100%). Dehydration was started in 50% ethyl alcohol. 10% increases of alcohol concentration were used in the dehydration steps. The filter membrane was removed from the holder, infilterated in LR write resin at room temperatures for 1 hr (two changes of LR white resis) and embedded in fresh accelerated LR white resin in 30 min. on ice. For gold-labelling, ultrathin sections were placed on and carbon coated copper grids. parlodion These grids were blocked with TBST/5% BSA buffer (pH 7.5) for 30 min to saturate nonspecific protein binding sites and then reacted for 1.5 hr with affinity purified FtsA specific antibodies (1 pg/ul) in dilutions of 1:50 in TBST/ 1% BSA buffer. In parallel, sample grids were processed with preimmune IgG antibodies (0.5 yg/ml) in dilutions of 1:25 as controls. After five subsequent washings in TBST/1% BSA buffer (5 min each), the sections were floated on goat anti-rabbit IgG 10 nm gold conjugate (Janssen's) in TBST/1% 41 BSA buffer. The grids were again washed 5 times in TBST/1% BSA buffer and rinsed in distilled water. Sections were stained in 1% uranyl acetate before being examined with a JEOL 100CX transmission electron microscope at 80 KV. Ultra thin sections of IPTG induced cells (YC200) were immunogold-labelled with FtsA-LacZ antibodies as described above. XVII. Preparation of cell extracts for enzyme assays. Cell extracts for CAT/fl-lactamase assays were prepared by the method of Lupski et al (72). Cells containing plasmid were inoculated in L broth supplemented with 0.2% glucose and ampicillin(50 yg/ml) or carbenicillin(25 pg/ml). An overnight culture was diluted 100 fold in fresh broth and grown to an 0D6Oo = 0.2 to 0.5 (Spectronic 20, Baush and Lomb). Six mis of cells were collected in a 15 ml corex tube by centrifugation at 7000 rpm for 10 min in Sorvell SS34 rotor. The cells were washed in 2 ml of 50 mM Tris, 30 pM DTT,pH 7.8, resuspended in 600 yl of same buffer and transferred to 1.5 ml Eppendorf tubes. The cells were frozen by placing at -70°C for 1 hr. Cells were then thawed and sonicated with 2 or 3 pulses of 10 sec with at least 15 sec rest between pulses using a MSE sonicator with a micro-tip. To remove cell debris, the cell extracts centrifuge at 4®C for 15 min. were centrifuged in an Eppendorf The supernatant was placed in another Eppendorf tube and kept on ice for subsequent enzyme assays or at 20°C for storage. 42 XVIII Enzyme assays. a. 3-galactosidase assay B-galactosidase activity was determined (ONPG) Miller(83). An aliquot of the sample to be assayed was (final NaH2POA-H20, volume 1 ml) according containing to o-nitrophenyl- galactoside buffer as a substrate using the method added to Z 60 mM Na2HPOA-7H20, 40 mM 10 mM KC1, ImM MgSO*, 50 mM 8-mercaptoethanol, pH If the sample consisted of intact cells, 40 ul CHC13 and SDS were added and vortex for 10 secs. of 20 ul 7.0. 0.1% Sample tubes are incubated in a water bath at 28°C for 5 minutes for equilibration. The reaction was started by adding 0.2 ml of 0NPG(4 mg/ml) and stopped by adding 0.5 ml of developed. was IM Na2C03 solution after sufficient yellow color had The optical density at both 420nm and 550nm for each tube determined and the units of P-galactosidase activity was calculated from the following formula: units = 1000 X (0D^2o " 1.75 X 0Dsso)/t X v X 0D&oo« Where t is length of reaction time in minutes and v is the volume of the enzyme extract used per ml of assay mixture. b . CAT Assay. Cell extracts as described in section XVII were used in the CAT assay. The reaction mixture was freshly prepared by dissolving 8 mg of DTNB (5,5'-dithiobis-2-nitro benzoic acid) in 2.0ml 1.0 M Tris-HCl, pH 7.8, adding 0.4 ml acetyl-CoA (5 mM) stock solution, and adjusting the total volume to 20 mis. 600 yl of the reaction mixture was placed in a reference cuvette and sample cuvette in a Beckman double beam recording Model 30 spectrophotometer. 20 yl of enzyme extract was 43 added to thereaction mixture and measured for 30 sec to 3 min using the spectrophotometer recorder to obtain the background activity. chloramphenicol recorded the A«l2 was adjusted to zero, then for To start the reaction, 12 yl of 5 mM was added. The increased absorbance about 5 min. The CAT enzyme at A* j.2 was specific activity (in nmol/min/mg) was determined by dividing nmol/min-ml by the protein concentration(mg/ml) as follows: nmoles/min/mg = AA*i 2 X 1/0.0136 X 1/mg protein C. fl-lactamase Assay. Cell extracts as described in section XVII were used. 600 yl of a reaction mixture consisting of 41.5 yg/ml cephaloridine (10~*M) in 0.1M phosphate buffer pH 7.0 was placed in a sample cuvette. Spectrophotometer was blanked against 0.1M phosphate buffer pH 7.0 and the A 2 5 5 was recorded for 30 sec. to get a base-line level. The absorbance of the cephaloridine mixture (A25s) was between 1.2 and 1.4. To start the reaction 20 yl of the enzyme extract was added to the reaction mixture and the A25s was recorded for approximately 5 min. A fall of the optical density from 1.4 to 0.6 would indicate complete hydrolysis. Specific nmole/min/mg protein) was calculated activity for B-lactamase (in by (AA2Ss/min) X 375/mg protein. An alternate substrate used was PADAC. Again a 600 yl reaction mixture was used consisting of 15.6 yg/ml PADAC (2.8 X 10_5M) in 0.05 M phosphate buffer, pH 7.0. The Specific activity PADAC hydrolysis was measured at As&&. for P-lactamase in nmole/min/mg protein was determined by (AAssa/min) X (1/0.0605/mg protein). 44 XIX. Protein Assay. Amount of protein was measured by the method of Bradford(15) using the Biorad Protein Assay dye reagent concentrate. Bovine serum albumin (Sigma) was diluted and used for protein standard concentrations. XX. Plasmolysis of bacterial cells Plasmolysis of bacterial cells were method of MacAlister and Rothfield(128). performed by a modified A strain AMC410(ftsZ84) grown in YET broth overnight at 30°C were diluted (1:100) in fresh medium. A portion of the growing cells at 30°C (0D6Oo = 0.2) were shifted to 42°C for 90 min. in a microfuge for 1 min. Culture was harvested by centrifugation The pellet was suspended in 0.1 volume of fresh culture medium at room temperature. suspension was plasmolyzed by adding equal A portion of this cell volume of plasmolyzing solution (26% suerose(wt/vol), 0.2 M cacodylate-Cl buffer, pH 7.2). These cells were kept at room temperature for 3 min before they were fixed by the addition plasmolyzing buffer. of equal volume of 4% formaldehyde in The cell suspension was allowed to stand for an additional 60 min. at room temperature, before being centrifuged for 30 sec in microfuge and being suspended in fresh plasmolyzing solution. Unplasmolyzed cells were also fixed with formaldehyde in parallel. Micrographs were taken with a phase contrast microscope (BH-2; Olympus, Splan lOOpl objective) with a 35 mm camera. Portion of the fixed plasmolyzed and unplasmolyzed cells were thin sectioned and immunogold-labelled as described in section XVI. RESULTS I. Isolation of FtsA-LacZ protein fusions. pRGC31 plasmids with mini-Mu, MudII1734, insertions were selected as Mu-ductants that were both amp*- and kanr and grew as light blue colonies on X-gal indicator plates. Approximately 1% of the total kanr colonies expressed B-galactosidase indicating an in-frame protein fusion with lacZ. Of these approximately one in fifty(2%) were found to be within the ftsA gene by restriction enzyme mapping. The mini-Mu insertion sites of four isolated ftsA-lacZ 6 550 plasmids are shown in Fig. In plasmid pYC40, MudII1734 transposon was inserted approximately bases from the start of the ftsA amino-terminal end. In plasmid,pYC39, the transposon insertion was approximately 650 bases, and in plasmids, pYC28 and PYC16, the mini-Mu was inserted 850 bases from amino-terminal end. Temperature sensitive ftsA mutant strains, TKF10 or YC100, remained temperature sensitive at the nonpermissive temperature(42°C) when the ftsA-lacZ fusion plasmids were introduced. fusions may plasmids, became retain some pYC39 and pYC40, filamentous filaments were functional at observed the were larger protein transformed permissive with the transformed with pYC39 and pYC40. slightly interactions. fusions, septation in AMC290. 45 wild When the the fusion into strain YC100 temperature type However, (30°C). strain, AMC290, it Some when Interestingly, plasmids encoding pYC16 and pYC28, did not effect 46 II. Expression of FtsA-LacZ fusion polypeptide in maxicells. The synthesis of FtsA-LacZ fusion proteins was confirmed by labelling the plasmid specified proteins using the maxicell labelling procedure(99,100). Autoradiograms of the [35S]methionine labelled polypeptides encoded by several plasmids in strain CSR603 is shown in Fig. 7. On this gel system, the FtsA protein migrates as a 48 kD polypeptide, FtsZ as a 45 kD polypeptide and 8-lactamase as a 28 kD polypeptide(Fig. 7, lane a, pRG31). All transformants with presumed ftsA-lacZ fusion containing plasmids synthesized large polypeptides migrating near the top of the SDS-PAGE gel with concomitant loss of the FtsA polypeptide band(Fig.7, lanesb,c,d). specified a new 142 kD fusion peptide. encoded 154 kD fusion peptides. Plasmid pYC40 Plasmids, pYC28 and pYC16 Plasmid pYC39 specified a hybrid peptide of 146 kD (data not shown). III. Cellular localization of the FtsA-LacZ protein. It was imperative to determine whether the amino-terminal end of the FtsA protein could localize similar to the FtsA protein. the FtsA-LacZ fusion polypeptide Native FtsA protein was labelled in maxicells containing pRGC31(Fig. 7). CSR603 strains containing pYC28, pYC40 or a control plasmid pRGC43 which encodes P-galactosidase and FtsA were also labelled. The labelled maxicells were fractionated into membrane and cytoplasmic fractions by centrifugation and part of the membrane fraction was further extracted with 1% Sarkosyl detergent to solubilize the inner membrane proteins. Table 3 presents the quantitative data as relative molar yields of labelled proteins from an electrophoresis autoradiogram . The 8-galactosidase peptide 47 specified by pRGC43 was localized in the cytoplasmic fraction with only a small contaminating amount visible in the membrane fractions. The FtsA protein, specified by both pRGC43 and pRGC31, fractionated almost equally between the cytoplasmic fraction and the total membrane fraction. FtsA Furthermore, approximately 50% of the membrane fraction of remained after Sarkosyl extraction. The FtsA-LacZ fusion proteins specified by plasmids pYC28 and pYC40 behaved identically to the FtsA protein. The presence of FtsA and FtsA-LacZ labelled proteins being found in both the soluble and membrane fractions is most likely a consequence of the maxicell protein labelling procedure. Labelled proteins are likely to be synthesized in excess, and maxicells are division arrested due to extreme chromosome DNA damage. Since the FtsA-LacZ fusion protein has P-galactosidase activity, this activity could be used to localize the FtsA-LacZ protein in wild type cells. We fractionated the membranes of strain AMC290 containing fusion plasmids by the procedure of Ishidate et al.(59). This procedure enables the isolation of unique membrane fraction called "outer membrane-light. (0Ml) membrane fraction. The which contains enzyme collected( activity in Fig. inner-murein-outer P-galactosidase activity isopycnic sucrose gradient was determined fractions the 8). membrane Most for the (80%) from the final different density of the (5-galactosidase preparation of the FtsA-LacZ fusion specified by pYC40 was found in the two fractions corresponding to 0M„(density 1.24 to 1.26) and 0ML(density 1.21-1.23). A small amount of LacZ specified P-galactosidase activity was found in the inner 48 membrane(IM) 1.12). fraction and slightly lighter fraction(density 1.16 to Similar results were obtained with cells containing fusion plasmid pYC16. IV. Overproduction and purification of FtsA-LacZ protein. In order to produce large quantities of the FtsA-LacZ hybrid protein, the 8.5 Kb BamHI fragment of plasmid pYC16(23) encoding the hybrid protein was subcloned into the expression plasmid pGW7(30). Plasmid pYC16 was partially digested with BamHl and the 8.5 Kb fragment was subsequently recovered from a preparative agarose gel and ligated into BamHl digested and dephosphorylated pGW7 DNA. One kan^ carbr colony contained a plasmid, designated pYC3, which had the 8.5 Kb insert in the correct orientation as determined by restriction enzyme digestion with BgllK Fig.9). When strain AMC290 shifted from 30°C to 42°C, (Alac) containing pYC3 are temperature transcription of the ftsk-lacZ DNA was induced resulting in increased fusion protein production. The level of hybrid protein production was measured by P-galactosidase specific activity. The optimal P-galactosidase activity was found after 2.5 hr at 42°C. These temperature accumulated up shifted pYC3 containing cells to 1% of the total cell protein as FtsA-LacZ protein. P-galactosidase activity was approximately 10 fold higher (7230 U/mg) after induction containing the (687 U/ml) and 20 fold higher than same cells original fusion plasmid pYC16(347 U/mg). The hybrid protein was purified from the soluble fraction of disrupted cells by two purification step: 40% (NH^)2S0A precipitation cut and affinity chromatography on p-aminobenzyl-8-D-thio- 49 galactopyranoside-agarose. In Fig. 10, the composition and degree of purity of the total soluble proteins, the 40% (NH«)2S0A fraction and the affinity-purified fractions separated by SDS-PAGE are shown. The 40% ammonium sulfate precipitant fraction retained approximately 90% of total 0-galactosidase activity and there was an enrichment of the hybrid protein (Fig. 10, lane D). The hybrid protein had the expected size compared to P-galactosidase molecular weight marker (Fig.10, lane E),and was identical to fusion protein specified by plasmid pYC16 (which had been labelled by maxicell labelling). polypeptide band, that was probably A 0-galactosidase a degradation product of the fusion protein, was always present in the affinity-purified protein fraction. This protein degradation may be due to enzymatic activity occurring during temperature affinity the purification which was performed at room (to increase the binding of the fusion protein to the matrix). The cleavage of fusion to P-galactosidase polypeptide can be expected because FtsA-LacZ fused protein is likely to have two distinct protein domains. V. Identification of wild type FtsA protein by Western blots. Polyclonal antibodies from rabbit against the FtsA-LacZ fusion protein antigen were prepared. The antiserum contained a significant titer of antibodies to both the FtsA-LacZ and LacZ determined by the ring preciptin tests and by dot blot analysis (data not shown). Western immunoblotting experiments were performed with an FtsA-LacZ immunoglobulin fraction (a final protein concentration of 1 mg/ml that was purified from serum using a protein A affinity column). 50 To test wild type FtsA protein cross reactivity with the FtsALacZ antibodies, the FtsA protein was [35S]methionine labelled using maxicell containing immunoblotting was plasmids encoding performed. FtsA before [3sS]methionine Western labelled FtsA polypeptides, visualized by autoradiography, was also found by Western blotting to be enzymatically 11B). labelled by FtsA-LacZ antibodies(Fig. As expected, both [3sS]methionine labeled fusion polypeptides specified by plasmids pYC16, PYC40 (Fig.llD, lane 3 & 4) and labeled 0-galactosidase specified by control plasmid pRGC43 also cross reacted with FtsA-LacZ antibodies (Fig. 11D, lane2). The FtsA-LacZ fusion proteins synthesized in maxicell appeared to be unstable because a strong antibody cross-reactivity band similar in mobility to 0- galactosidase was observed upon Western blotting.' Thus, the results indicated FtsA-LacZ that the polyclonal antibodies from protein antigen was specific for FtsA, FtsA-LacZ and LacZ polypeptides. Additional immunoreactive bands which stained less intensely (Fig. 1IB, lane 1 & 2) were visible in total cell extracts. One at 37 Kdal and several small peptides at the bottom of the Western blot. These bands,however, were not observed with the soluble protein fraction probed by Western blotting with FtsA-LacZ antibodies. That is, these extraneous bands appeared in only the membrane fractions. These bands could be the result of cross reactivity of FtsA antibodies with other membrane proteins, modified FtsA protein or its breakdown products. Nonspecific binding could not be completely excluded because preabsorbtion to remove nonspecific antibodies were performed with a cleared lysate. However,immunoglobulins from pre-immune serum 51 did not show any reactivity in Western blots of membrane proteins under the same experimental conditions(data not shown). To determine whether the FtsA protein synthesized in wild type cell could be detected with the FtsA-LacZ antibody preparation,total cellular polypeptides from a Lac deletion strain AMC290 were separated on SDS-PAGE and Western blotted with several concentration of FtsALacZ antibodies (data not shown). The amount of FtsA within a wild type cell was apparently too small to be detected in a total cell extract. Wild type FtsA protein was detected in an immunoblot of protein from a 40% ammonium sulfate pelleted fraction of AMC290/pYC3 which was temperature peptide (Fig. 12). shifted to overproduce the FtsA-LacZ fusion Apparently, this plasmid containing strain either synthesized more FtsA or wild type FtsA became more stable because of the FtsA-LacZ fusion protein synthesis. However, this experiment gave us confidence that we could detect wild type FtsA if a cell fraction was enriched for FtsA. VI Cellular localization of FtsA protein. Membrane fractionation studies with FtsA-LacZ expressing strains revealed that 0-galactosidase activity was associated with membrane fractions corresponding membrane light (OM*,). to outer membrane heavy(0M„) and outer Previous studies above demonstrated that we can detect FtsA protein by Western blotting. Thus,to verify that native FtsA protein indeed was enriched and localized to membranes in wild type E. coli cells two experimental approaches using the FtsA specific polyclonal antibodies were performed: A. Western immunoblots of cell fractions and B. Immunoelectron microscopic localization. 52 A. Western blots of cell fractions. AMC290(Alac74) cells were fractionated into cytosol and envelope membrane fractions first by centrifugation. fraction were separated by SDS-PAGE, The protein from both blotted onto Nytran membrane filters and probed with FtsA-LacZ antibodies. A band in the envelope fraction, corresponding in molecular weight to native FtsA, interacted with FtsA-LacZ antibodies but not in the soluble fraction (Fig.13, lanes 5,6,8,9). Increased g-mercaptoethanol concentration from 1 to 5% in PAGE buffer in attempts to remove the cross antigenic smearing that was found with SDS-PAGE of the envelope fraction( Fig 13) resulted in the labelling of two bands (60Kdal and 52Kdal) above the 48K FtsA band. In addition, another and band (37Kdal) below the FtsA band was labelled in SDS-PAGE of envelope and soluble fractions(Fig. 14, lane E,F). Two bands corresponding to 33Kdal and 23Kdal polypeptides and one immunoreactive band at the bottom of the gel were also labelled in SDS-PAGE of the envelope protein fraction. The later two bands, 33Kdal and 23Kdal, because are probably these intensively, as degradation products polypeptide bands [35S]methionine are of FtsA. observed, labelled This although polypeptides is less of maxicell into specific containing plasmid pRGC31(specifying FtsA). The cell envelope was further fractionated membrane domains using neither lyzozyme or EDTA but French pressure cell lysis followed by sucrose floatation steps and isopycnic sucrose gradients as described previously (section III). 0MH , 0ML and IM membrane fractions were separated on SDS-PAGE(10-30%) and the proteins 53 were electro-transferred to Nytran sheet. result of Fig. 13 and 14, show the Western blotting with FtsA-LacZ antibodies. In Fig. 13 (lanes 1,2,3,4) the data revealed that native FtsA cross reactivity to FtsA-LacZ antibodies were found in QM„ and 0ML fraction of AMC290 (0D6OO=1.6). Thisresult studies. is consistent with the FtsA-LacZ Similarresults immunoblotting membrane were obtained after fractions of YClOO(ftsAlO) shifted to 42°C for 2 hrs. fusion Western strain that was This indicated that ftsAlO does make a cross reactive protein which is incorporated into the envelope but probably is not functional. When buffer, fraction B-mercaptoethanol the cross but also concentration reactivity in IM to FtsA was fraction was found (Fig. 14, increased not in only lane PAGE in 0ML A,B,C,D) of AMC290(0D6Oo=0.4). The labelling of the cross reacting peptides were due to antibody specificity conjugant labelling because immunoblotting and not due to any spurious enzyme- similar results were obtained when Western was performed with a different secondary antiserum, 125I-labelled goat anti-rabbit IgG under same experimental conditions. Fig 15 presents the SDS-PAGE profile of Coomassie blue stained polypeptides which Western blotted and presented in Fig. 14. The membrane fractions additional immunoreactive (Fig.14,lanes To exclude the contained labelled bands similar to labelled bands observed in total membrane fraction and the E,F). A,B,C,D) possibility soluble fraction thatthe appearance of (Fig 14, these additional bands was due to nonspecific binding (for example, cross reactivity to the major outer membrane protein, OmpA(37Kdal)), FtsALacZ IgG fraction was preabsorbed twice with a lysate containing cell 54 membrane from SGO fraction nonspecific antibodies. fraction is shown additional bands (see Material Methods) to remove The result of Western blotting with this IgG in Fig. and and also 16. of The the labelling FtsA band intensity of the decreased. These results,although not conclusive, indicated that the several additional immunoreactive bands are not result cross reactivity of FtsA-LacZ antibodies with any major E. coli membrane proteins. It is more likely they are precursors, breakdown products or modified forms of FtsA protein. The presence of 4M urea in the sample solubilization buffer was required to cleanly detect the FtsA protein by Western blotting. If urea was left out of sample buffer, FtsA band was not visible and cross immunoreactive material migrated near top of gel. The urea effect, probably indicates that FtsA aggregates with other membrane protein or itself even in the presence of 1% SDS. (Increased SDS in solubilization buffer resulted in the inability of FtsA-LacZ antibodies to cross-react with gel proteins.) B. Immunogold labelling and electron microscopy. a. Wild type cells. Direct immuno-electron antibody using colloidal gold goat to microscopic anti-rabbit mark E. coli observations immunoglobulin cell bound of FtsA conjugated with FtsA antibody were performed. Basically, ultrathin sections of AMC290 cells were isolated by low temperature embedding of cells in LR white accelerator at 4°C and reacted with FtsA-LacZ antibodies. resin with Binding of 55 these antibodies was detected with goat anti-rabbit IgG conjugated to gold particles. In fig.17(A,B,C,D) aggregates in the the gold particles were found envelope with few being found in mostly as the central cytoplasmic region. No gold particles were found in samples treated withpreimmune antibodies(Fig.17; E,F) instead of FtsA-LacZ antibodies. The gold particles appeared to be aggregated at sites that appear to be septa or one polar end of the cell. As a control, 0-galactosidase was induced with IPTG (YC200,lac+) and the cells were thin section and treated as above. Most of the gold particles were found throughout the cytoplasm (Fig.17;G,H). b. Plasmolyzed cells. Potential septation sites can be identified by phase contrast microscopy and cells (78). sites and by electron microscopy using plasmolyzed bacterial The resulting plasmolysis bays define potential septal a adhesion zone. cellular region consisting of inner-outer membrane To determine whether inner-outer membrane regions within Fts filaments could be visulized, cells were plasmolyzed and electron micrographs of ultrathin sections were analyzed. As described previously, ftsZ84(ts) cells at elevated temperature results in the formation of long filaments without any visible septa. When ftsZ(AMC419) mutant filaments were plasmolyzed, multiple plasmolysis bays were visible along the length of the filament (Fig. 18). various sized nonseptal plasmolysis bays appeared at a The near cell length intervals expected if bays define potential septation sites. 56 However, the number and distance of the plasmolysis bays along the length of the filaments varied somewhat with sucrose concentration and with growth conditions. bays were ftsl(ts) Similar distribution patterns of plasmolysis observed by phase filaments which contrast also have microscopy some of ftsAlO(ts) visible or constrictions identifying septal sites(data not shown). To study distribution of FtsA protein in ftsZ nonconstricting filamentous cells direct immunoelectron microscopy was performed. The electron micrographs (Fig. 19) show that gold particles representing antibody bound FtsA were found mostly as aggregates at intervals along the envelope of the nonseptal filaments. Immunoelectron micrography of plasmolyzed ftsZ filaments, to locate inner-outer membrane regions, were also analyzed under same experimental conditions. particles were The micrograph in Fig. 20 reveals that gold distributed as aggregates at junction sites defined by the plasmolysis bays. inner-outer membrane Furthermore, the gold particles were clustered at regular intervals along the length of the filament similar to the non-plasmolysed cells(Fig. 19). These results confirm that FtsA proteins are associated with potential septation sites. VII. Investigation of ftsZ gene expression. A. Promoter gene fusions and quantitation assays. The vector used in all of the promoter fusion experiments was pKK232-8 (16). It contains a chloramphenicol acetylase(CAT) structural gene whose natural promoter has been deleted( Fig. The CAT 21). gene in this vector can be expressed from a promoter inserted into an upstream restriction enzymes polylinker site. Translation 57 proceeds only from a start site immediately flanking the gene. The amount of CAT activity in a cell harboring the plasmid is thus an accurate index of the promoter strength of DNA fragments containing promoters which are inserted into the polylinker site. The constitutively expressed (J-lactamase gene on the plasmid provides a selectable marker and an internally expressed gene for normalization. That is, the ratio of CAT activity to (3-lactamase activity gives a measure of the strength of the inserted promoter that is independent of plasmid copy number and overall metabolic activity of the host cell. The different ftsZ promoters (PziPzaPza) and ftsA (pA) promoter regions were subcloned into pKK232-8 polylinker region and then CAT/fJlactamse specific activity in wild type strain (AMC290) background (Table 4) were determined. The potential proximal promoters(110), Pzx and Pz2, within the Hindlll-EcoRl DNA fragment of the plasmid pYC580 was poorly expressed in vivo being approximately 21.8 X 10-3 units. This is consistent with low level of ftsZ expression from a AenvA(77), that only partial complemented a ftsZ84(ts) mutant. In contrast to PziPza. the promoter Pz3 within the coding sequence of ftsA, as in PYC530, showed a high level of promoter activity, approximately 235.2 X 10-3 units. If the' ftsA gene promoter, PA , was fused to Pz3 (BamHl- Bglll), as in pYC500, then the promoter activity increases slightly indicating a weak ftsA. promoter. This indicates that the ftsk promoter is weak and contributes little to the transcription from Pa Pz3-CAT fusion. The total promoter activity from pYC620 was lower than the sum of the separate included with activities of PzaPzaPzi. as Pz3 and PziPz2 - in pYC600, the promoter When PA was activity was 58 increased by 1.8 fold but still lower than the level of transcription from pYC53(P23). Lutkenhaus and his colleagues!.'75) have shown that the presence of part of ftsQ structural gene, as in pYC600, results in the decreased ftsZ expression and TnlO insertion into ftsQ gene recovers ftsZ gene expression enough to suppress the SOS induced lethal filamentation of the Ion mutant. However, the maximal expression of ftsZ either with a single copy phage vector (130), or with a multicopy plasmid(122) has been shown to require approximately 2 Kb of DNA upstream of the ftsZ gene, including ftsQ promoter. B. Effects of FtsA, FtsQ, FtsZ, and DnaA on ftsZ expression. Expression of ftsZ has been cited to be negatively regulated at the transcriptional level by FtsI, FtsQ, FtsA and FtsZ itself(36). Temperature shift experiments with temperature sensitive mutants of ftsQ, ftsA, and ftsZ were performed to assess whether any ofthe fts gene products regulate the transcription of another fts cell division gene. Since temperature sensitive fts mutants result in an inactive protein product at the nonpermissive temperature, the effect on transcription is easily determined by measuring the CAT/8-lactamase specific activities, using the promoter fusion plasmids constructed above. Table 5 shows that FtsZ temperature inactivation increased CAT expression from both PAPZ3 and Pz3. 1.5 to 3 fold. Wild type The level of increase was about control also activity from PAPZ3 and Pz3 by 3 to 4 fold. increases the promoter Autoregulation of ftsZ expression can't be completely excluded although the data suggest that 59 the increased CAT expression by FtsZ inactivation could result from the temperature shift from 30°C to 43°C. LacZ-transcriptional studies which The data is comparable to showed no apparent ftsZ autoregulation (R. Gayda, unpublished). FtsQ inactivation (Table 6) had stimulating effects both on PAPz3 promoter combination and on Pz3 promoter alone. The FtsA inactivation (Table 7) had a slight decreasing effect on Pz3 promoter with only little compared with wild type control. effect on PzaPZi promoter when A low level of CAT expression was obtained because of the temperature sensitivity of the ftsA mutant strain even in the presence of high salt. These data are opposite to the finding(36) that transcription of ftsZ is increased as much as 10 fold when cells have a nonsense mutation in the chromosomal copy of ftsA gene. On the other hand, expression of ftsA activation of transcriptionbecause may be there is a evidence supporting that the controlled the by FtsA FtsZ gene proteinthrough expression of plasmid pRGC43 was no longer sufficient to complement ftsk mutants when the ftsZ gene was inactivated by the mini-Mu insertion (R. Gayda, unpublished). Possible dnak consensus sequence within ftsZ promoters(PzaPzi) led us to study if dnak effected the expression of ftsZ gene. Table 8 shows that dnak temperature inactivation increased CAT activity nearly 2 fold from both PAPZ3 and Pz3, suggesting that dnak regulation may link septation with DNA synthesis. DISCUSSION Previous genetic studies have postulated that the function of FtsA protein is both regulatory and structural in septum formation(115-118).The purpose of this study was to investigate the structural involvement of FtsA in septum formation. The establishment of the cellular location of FtsA is important because current data (10,29,31,41,65,119) strongly proposed interactions among the components of the division machinery. within a specific membrane domains Thus the locating FtsA will make possible the determination of the specific functions or biochemical activities of FtsA in septation and will begin to unravel the ordered morphogenic molecular mechanisms involved in the septation process. Evidence obtained that FtsA protein is acting as an inner-outer membrane junction protein are as follows. First, FtsA-LacZ fusion proteins demonstrated that the amino terminal end of the FtsA protein can localize FtsA-LacZ hybrid protein to the cell envelope (Fig. 6 and Fig. 7). Furthermore, FtsA-LacZ translational fusions behaved like FtsA protein when radioactively labelled using the maxicell technique and when fractionated into membrane and cytoplasm (Table 3). Second, wild type dividing cells synthesizing the FtsA-LacZ hybrid proteins, when the membranes were fractionated by a modified sucrose equilibrium gradient exhibited, 3-galactosidase corresponding to OM„ and 0ML (Fig. inner-outer membrane fusions(59). were made from purified experiments with these enzyme 9). in fraction The 0ML fraction contains Third, when polyclonal antibodies FtsA-LacZ antibodies 60 activities proteins, on cell Western-blotting membrane fractions 61 demonstrated that native FtsA was found fractions (Fig. 13). concentration of localized to 0M„ and 0ML (Other experiments conducted with increased 0-mercaptoethanol in SDS-PAGE showed that the FtsA can also be detected in inner membrane(IM) fraction (Fig 15,16)). Fourth, direct immunoelectron microscopic localization confirmed the specific localization of FtsA protein at septation sites within the wild type cells. The immunogold-labelling of thin sections of filamentous cells indicated an enrichment of FtsA protein at potential septation sites which were identified as inner-outer membrane junction sites by plasmolysis. Thus, our model is that FtsA along with other septation proteins forms part of a "septalsome" and modulates the sequence of biochemical core which initiates changes that lead to formation of the division septum. Ishidate et al. (59) have reported that the 0ML membrane fraction is involved in murein synthesis and the translocation of newly synthesized lipopolysaccharides. Recently, MacAlister et al. (78) have demonstrated that 0ML fraction also contains the periseptal annuli which flank the constriction sites around the potential septum as an inner-outer membrane gasket to compartmentalize the periplasmic space between the dividing cells. Similarly, it has been reported (4,97) that the penicillin binding proteins (PBPs) are located at discrete zones within the cell envelope, an inter-membrane fraction probably corresponding to innerouter membrane adhesion sites or periseptal annuli(5,78). PBPs are a set of actual enzymes that catalyze the insertion of new material into the peptidoglycan sacculus and are engaged in the control of cell elongation(106) and cell division(14,63). One of them, PBP3(ftsI) gene 62 product, is a known septation proteins which has transglycosylase and transpeptidase enzymatic synthesis septum and activities formation. which are involved Overproduction of in murein inactive PBP3 results in inhibition of cell septation implying that PBP3 is part of a protein complex in vivoi15B). FtsA mutants have increased resistance to 0-lactam induced lysis and decreased binding of [12SI] ampicillin to PBP3. FtsA. an This supports a possible interaction of PBP3 with In fact, it has been suggested that FtsA functions as part of active multimeric biosynthesis for complex septum by regulating formation(23). or directing Furthermore, murein indirect evidence, which comes from murein synthesis studies by topographic autoradiography, show that increased incorporation of radioactive diaminopimelic acid in the central zone representing the present or future site for septum formation (127). Observations(data not shown), that fusions specified pYC39 or pYC40 caused some filamentous cell growth even in wild type strains also supports a functional role of FtsA as a component of multimeric complex in ^septation. FtsA-LacZ fusions may be competing with wild type FtsA protein that may be functioning as an oligomer or mixed oligomer with other septation proteins. The fractions localization of like FtsA protein indicates native FtsAlO protein to 0M„ and 0ML membrane that FtsAlO probably leads to a nonfunctional complex due to an altered protein structure. FtsZ probably is also involved in the multienzyme complex. highly expressed promoter(124) death. LacZ-FtsZ protein under control of the A lac when induced by IPTG(123) result in filamentation and Whereas, overproduction of FtsZ results in early cell 63 septation and minicell between specific formation(124). alterations of FtsZ This functional similarity and overproduction of FtsZ supports an involvement of some type of stoichiometric regulation of this complex division machinery. The ftsA gene consists of 420 residue amino acids(95) with a calculated molecular weight of 45.4 Kdal. The FtsA protein has been identified as a 50 Kdal polypeptide (76) on SDS polyacrylamide gels of extracts of in vivo transducing phage. labelled cells with ftsA on plasmid or A This 10% discrepancy of this protein on SDS-PAGE gels that has been reported (95) is probably due to fact that it is a membrane protein. The FtsA gene product in relationship to FtsZ is synthesized much less molecules (a of few molecules(53) FtsA per cell of FtsA (52,76): to maximally based on a hundred intensity of autoradiograms(76), FtsA is expressed as much as 10 fold less than FtsZ, and based on the amount of 0-galactosidase enzyme activities of FtsA transcriptional fusions compared with FtsZ transcriptional fusions FtsA is expressed as much as 40 fold less than FtsZ. amount of FtsA protein within The low cells could be because FtsA protein is synthesized at a low level, or because FtsA is degraded at a high rate. Our results indicate that both are likely occurring. Assuming that the specific activity of native 0-galactosidase is very similar to that of fused FtsA-LacZ protein activity (where 0.06 units of 0galactosidase activity corresponds cell(92)). to 0.3 monomers of protein per Assuming that the cell on average contains 50 copies of fusion plasmid per cell (1 transcript or 1 translation per transcript per plasmid per generation) We estimate that there are 20 to 200 64 (probably averaging around 50) FtsA protein molecules per cell from the amount of P-galactosidase enzyme activities of FtsA transcriptional and translational fusions. Promoter strength assay (Table 4)of ftsA promoter and the immunoelectron microscopic localization (Fig. 17) are consistent with this low level expression of ftsA. containing fusion protein Immunoblot(Fig. 11D) of extracts specified by pYC40 and enriched fusion protein specified by pYC3 also strongly suggest that FtsA protein is unstable because only g-galactosidase part of hybrid proteins were intensely antibodies cross-reacted with FtsA-LacZ whereas no immunoreactive band corresponding to the FtsA part of fusion protein was observed. The difficulty in identifying FtsA protein on immunoblots even when cells carrying ftsA multicopy plasmid could be ascribed to this small amount of FtsA protein. A 10 fold increased antibody concentration did not increase the sensitivity of FtsA antigenicity in the immunoblots under same experimental condition. Interestingly, the amount of the native FtsA protein was sufficient to be detected in immunoblot (Fig. 12) when 40% (NH*)2POA precipitate of FtsA-LacZ hybrid proteins enriched cell extracts were analysed. This observation suggests that overproduced FtA-LacZ protein could act as a positive regulator of ftsA expression or remove its negative regulator. increase the stability Alternatively, FtsA-LacZ hybrid protein could of the native FtsA protein by a mixed multimeric formation or autoregulation. Conditional temperature sensitive mutants of ftsA form long multinucleated non-septated filaments that have slight indentations at 65 the septation sites. This mutant phenotype plus other genetic studies(9,35,76) suggest that FtsA protein acts after the indentation of the new analysis(113) septation of cell sites. Morphogenic division mutants and have physiological proposed that the morphogenetic pathway is in the order of FtsZ-FtsQ-FtsA,FtsI. Subcloning, (95,96,109,129,130) ftsk-ftsZ) form an promoter fusionassay, and DNA sequencing have confirmed that contiguous fts genes (ftsQatypical operon with overlapping transcriptional units that transcribe in the same direction from the promoters (Fig. 1) locating upstream of each of the genes. terminators between the genes. There are no transcription This closely packed organization may imply that the variable levels of coupled expression of these genes are critical to the ordered functions of cell septation gene products. The observation that the presence of the ffindll-EcoRl DNA sequences decreased the ftsZ expression from Pz3 by 2 to 4 fold (Table 4) suggests that the normal chromosomal location of ftsZ genes for proper functioning of the gene is critical and/or mutual regulation. to their coordinate expression A report(133) that integration of a A phage that carries a wild type ftsA sequence into the attA site of a ftsk mutant, can only partially complements this ftsk mutant, point to an importance of the neighboring DNA sequences. In vivo quantitative promoter strength assays (Table 4) of the ftsk and ftsZ promoters in BanMl-EcoRl fragment(2.2Kb) subcloned in promoter probe vector pKK232-8 relatively weak promoter. confirms that ftsk promoter is a The potential proximal promoter PZi and Pz2 within the Hindlll-EcoRl fragment was also poorly expressed in vivo compared to the distal promoter Pz3 in Bglll-Hindlll region. A 66 comparison of the homology scores of known promoter sequences -35 and -10 region to the ftsZ promoter sequences gave the following scores: PZi= 53.3%, Pz2=45.6% and Pz3 = 37.3%. limit for an effective promoter. vivo data together controlled whereas suggest Pz3 that must be A score of 45% is the lower These homology scores plus our in PZ1 and Pz2 positively may be controlled negatively to be an effective promoter. There is some disagreement of our expression data with expression data published by Sullivan and Donachie (109) and by YI and Lutkenhaus (130). However, there is also disagreement between these two published papers. The differences suggest that this DNA region may be highly regulated by multiple factors linked to cellular events and by multiple interactions to control the variable levels of coupled expression at different times in the cell cycle. The differences are also likely to be the result of our plasmid constructions and the interaction of DNA sequences around these promoters. Expression study (Table 7) with temperature sensitive mutant ftsA showed that FtsA appears to increase the ftsZ expression from Pz3 at transcriptional level. Interestingly, there is an observation that ftsk expression may be controlled by FtsZ protein through activation of transcription because the ftsk gene of plasmid pRGC43 is no longer able to complement an ftsk mutant when the ftsZ gene was inactivated by the mini-Mu insertion (R. Gayda, unpublished). On the other hand, ftsZ expression(Table8) may be regulated negatively by dnak protein replication. DnaA protein has been shown to autoregulate its own synthesis is an and essential important control for initiation of chromosome element of E. coli origin 67 region(43). the Consensus sequences for DnaA binding have been found in regulatory regions of pyrBl, argFl, polA, uvrA and dam genes (111). The promoter expression data plus the DnaA consensus binding sequence within ftsZ promoters (PZ2PZ1) suggest that dnaA regulation of ftsZ expression may link septation with DNA synthesis. Protein sequence of FtsA(95) doesn't contain a typical leader signal peptide, that is present in precursors of most periplasmic and outer membrane proteins. This indicates that localization of FtsA into specific inner-outer membrane junction sites may occur through a different mechanism by which an internal protein sequences of FtsA is sufficient for proper insertion at a particular cellular location. The inner-outer membrane fraction is likely to be a labile structure. The membrane fractionation procedure employed neither lysozyme or EDTA treatments in order to preserve envelope structures. Furthermore, this lability probably explains FtsA-LacZ and FtsA being found in the 0M„ membrane fraction(Fig. 8 and 13). This is supported by the report(A) that PBPs are localized in the intermembrane fraction under the mild conditions for cell rupture and usually found in both the IM and OM membrane fractions when the inner-outer adhesion sites are destroyed by the cell breakage and fractionation procedures. Immunoblot analysis with membrane fractions isolated from sucrose equilibrium gradients indicates that the FtsA is localized to the specific domain of the envelope, sites. 60Kdal, evidence inner-outer membrane junction However,immunoreactive bands in addition to FtsA antigen at 52Kdal, 33Kdal, exclude the 23Kdal were observed. possibility that Several pieces of nonspecific binding is 68 responsible for the appearance of these immunoreactive bands. 1. Preimmune serum did not show binding on Western blots under same experimental conditions. corresponding [35S] 2. Two of the bands were also detectable as labelled bands, but specifying plasmid proteins were labelled Instability of FtsA protein suggest less intensely, when FtsA by maxicell procedure. that 33Kdal and 23 3. Kdal polypeptides are probably FtsA breakdown products or modified FtsA present in the membrane. The report that outer membrane protein OmpT is a protease(49) may support this. 4. Two times preabsorption of FtsA-LacZ antibodies fraction with lysate containing total membranes, to remove antibodies reacting with the major membrane proteins of E. coll, decrease FtsA antigenicity but did not eliminate these immunoreactive bands. The mechanisms used by the cell to identify the proper site and to localize the cell division machinery at this site are unknown. Rothfied's group(25) has proposed a model that nascent annuli are generated from a previous periseptal annuli acting as a boundaries of tseptation site and then laterally displaced till it reaches a midpoint between a cell pole and the generating periseptal annuli. Phase contrast and electron micrographs demonstrate the patterns of localized plasmolysis bays of periseptal annuli at septation sites and annular adhesion zones at nonseptal locations corresponding to future sites of cell division. Similar patterns phenotype properties of localized in different plasmolysis fts cell bays plus similar division mutants (.ftsZ, ftsk, ftsl) ( data not shown) suggest that FtsZ function, leading to the initiation of septal invagination, appears to be activated after 69 the maturation and localization of nacent periseptal annuli. The fact that ftsZ84 filaments after temperature shift back to 30°C, recovers cell septation quicker than model. However, the ftsA or pbpB possibility of filaments supports FtsZ protein is involved this in localization of the septation sites must also be considered because of minicell formation localization by due to immunogold increased electron FtsZ proteins. microscopy near FtsA's inner-outer membrane junction sites, when visualized by plasmolysis bays, suggests another possible role of nascent annuli. It is probable that the maturation of the periseptal annuli mediates localization of the septation proteins to the proper cell locations. Thus, our data supports that the septation proteins form a core, part of an hypothesized "septalsome", a multienzyme-protein complex at septation sites. Formation of this multimeric complex is probably modulated by multiple factors linked to several metabolic processes. Multiple interactions septation proteins appear to in the be involved septalsome which in activation of the intern modulates the enzymatic activities of the septation steps at precise times during the cell division cycle. LITERATURE CITED 1. Allen, J.S., C.C. Philip, R.A. Walker. 1974. Regulation and phenotypic analysis of nucleated filament-forming Bacteriol. 117: 978-986. Gustafson, R.G, Allen, and J.R. of bacterial cell division: genetic temperature-sensitive, multi mutants of Escherichia coli. J. 2. Anba, J., A. Bernadac, J. Pages, and C. Lazdunski. 1984. The periseptal annulus in Escherichia coli. Biol. Cell. 50: 273278. 3. Bachmann, B.J. 1983. Linkage map of Escherichia coli K-12, edition 7. Microbiol. Rev. 47: 180-230. 4. Barbas, J.A., J. Diaz, A. Rodriguez-Tebar, and D. Vazquez. 1986. Specific location of penicillin binding proteins within the cell envelope of Escherichia coli. J. Bacteriol. 165: 269275. 5. Bayer, M.H., G.P. Costello, and M.E. Bayer. 1982. Isolation and partial characterization of membrane vesicles carrying markers of membrane adhesion sites. J. Bacteriol. 149: 758-769. 6. Bayer, M.E., M.H. Bayer, and O.A. Lunn, and V. Pigiet. 1987. Association of thioredoxin with the inner membrane and adhesion sites in Escherichia coli. J. Bacteriol. 169: 26592666. 7. Beall, B. and J. Lutkenhaus. 1987. Sequence analysis, transcriptional organization, and insertional mutagenesis of the envA gene of Escherichia coli. J. Bacteriol. 169: 54095415. 8. Beck, B.D., and J.T. Park. 1976. Activity of three murein hydrolases during the cell division cycle of Escherichia coli, as measured in toluene-treated cells. J. Bacteriol. 126: 1250-1260. 9. Begg. K.J. and W.D. Donachie. 1985. Cell shape and division in Escherichia coli: experiments with shape and division mutants. J. Bacteriol. 163: 615-622. 10. Begg, K.J., B.G. Spratt, and W. D. Donachie. 1986. Interaction between membrane proteins PBP3 and RodA is required for normal cell shape and division in Escherichia coli. J. Bacteriol. 167: 1004-1008. 11. Begg, K.J., G.F. Hatfull, and W.D. Donachie. 1980. Identification of new genes in a cell envelope-cell division gene cluster of Escherichia coli: cell division gene ftsQ. J. Bacteriol. 144: 435-457. 70 71 12. Belhumeur, P., and G.R. Drapeau. 1984. Regulation of cell division in Escherichia coli: properties of new ftsZ mutants. Mol. Gen. Genet. 197: 254-260. 13. Boer, P.A.J., R.E. Crossley, and L.I. Rothfield. 1988. Isolation and properties of minB, a complex genetic locus involved in correct placement of the division site in Escherichia coli. J. Bacteriol. 170'. 2106-2112. 14. Botta, G.A. and J.T. Park. 1981. Evidence for involvement of penicillin-binding protein 3 in murein synthesis during septation, but not during cell elongation. J. Bacteriol. 145: 333-346. 15. Bradford, M.M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72: 248-254. 15B. Broome-Smith, J.K., P.J. Hedge, and B.G. Spratt. 1985. Production of thiol-penicillin-binding protein 3 of Escherichia coli using a two primer method of site-directed mutagenesis. EMBO J. 3:231-235. 16. Brosius, J. 1984. Plasmid vectors for the selection of promoters. Gene 29: 151-160. 17. Burnette, N.W. 1981. "Western blotting": Electrophoretic transfer of proteins from sodium dodecyl-sulfatepolyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radionated protein A. Anal. Biochem. 112: 195-203. 18. Burton, P. and I.B. Holland. 1983. Two pathways of division inhibiton in UV-irradiated E. coli. Mol. Gen. Genet. 190: 309-314. 19. Canepari, P., G. Botta, and G. Satta. 1984. Inhibition of lateral wall elongation by mecillinan stimulates cell division in certain cell division conditional mutants of Escherichia coli. J. Bacteriol. 157: 130-133. 20 . Castilho, B.A., Olfson, P. and M. Casadaban. 1984. Plasmid insertion mutagenesis and lac gene fusion with mini-Mu bacteriophage transposons. J. Bacteriol. 158: 488-495. 21. Chakraborti, A.S., K. Ishidate, W.R. Cook, J. Zrike, and L.I. Rothfield. 1986. Accumulation of murein-membrane attachment site fraction when cell division is blocked in IkyD and cha mutants of Salmonella typhimurium and Escherichia coli. J. Bacteriol. 168: 1422-1429. 72 22. Chang, C-F., H. Shuman, and A.P. Somlyo. Electro probe analysis, X-ray mapping and.electron energy-loss spectroscopy of calcium, magnesium, and monovalent ions in log-phase and in dividing Escherichia coli B cells. J. Bacteriol. 167% 935939. 23. Chon,Y. and E. Gayda. 1988. Studies with FtsA-LacZ protein fusions reveal FtsA located inner-outer membrane junctions. Biochem. Biophys. Res. Comm. 152% 1023-1030. 24. Corton J.C., J.E. Ward, Jr., and J. Lutkenhaus. 1987. Analysis of cell division gene ftsZ(suIB) from gram-negative and grampositive bacteria. J. Bacteriol. 169% 1-7. 25. Cook, W.R., F. Kepes, D. Joseleau-Petit, T.J. MacAlister and L.I. Rothfield. 1987. Proposed mechanism for generation and location of new cell division sites during the division cycle of Escherichia coli. Proc. Natl. Acad. Sci. U.S.A. <54: 71447148. 26. Cook, W.R., T.J. MacAlister, and L.I. Rothfield. 1986. Compartmentalization of the periplasmic space at division sites in gram-negative bacteria. J. Bacteriol. 168% 14301438. 27. D'Ari, R., A. Jaffe, P. Bouloc, and A. Robin. 1988. Cyclic AMP and cell division in Escherichia coli. J. Bacteriol. 170% 6570. 28. Davie, E., K. Sydnor, and L.I. Rothfield. 1984. Genetic basis of minicell formation in Escherichia coli K-12. J. Bacteriol. 158% 1202-1203. 29. Descoteaux, A. and G.R. Drapeau. 1987. Regulation of cell division in Escherichia coli K-12: probable interactions among proteins FtsQ,FtsA and FtsZ. J. Bacteriol. 769:11938-1942. 30. Deutch, A.H., C.J. Smith, K.E. Rushlow, and P.J. Kretschmer. 1982. Escerhichia coli delta*-pyrroline-5-carboxylate reductase: gene sequence, protein overproduction and purification. Nucl.Acid.Res. 23% 7701-7714. 31. Disabarro, A.G., R. Prats, D. Vasques, and Air Tebar. 1986. Activity of penicillin binding protein 3 from Escherichia coli. J. Bacteriol. 168% 194-206. 32. Donachie, W.D., 1979. The cell cycle of Escherichia coli, pp.1135. In J.H. Parish(ed.), Developmental biology of prokaryotes. Blackwell Scientific Publications, Ltd. Oxford. 73 33. Donachie, W.D. and A.C. Robinson. 1987. Cell division: parameter values and the process, pp.1578-1593. In Escherichia coli and Salmonella typhimurium cellular and molecular biology. ( F.C. Neidhart, ed. in chief). American Society of Microbiology, Washington D.C. 34. Donachie, W.D., and K.J. Begg. 1970. Growth of the bacterial cell. Nature (London). 227: 1220-1224. 35. Donachie, W.D., K.J. Begg, and J.F. Lutkenhaus, G.P.C.Salmond, E. Martinez-Salas and M. Vincente. 1979. Role of the ftsA gene product in control of Escherichia coli cell division. J. Bacteriol. 140: 388-394. 36. Donachie, W.D., K.J. Begg, and N.F. Sullivan. 1984. Morphogenes of Escherichia coli, pp.27-62. In R. Losick and L. Shapiro(ed.), Microbial Development. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y. 37. Dopazo, A., A. Tormao, M. Aldea, and M. Vincente. 1987. Structural inhibition and reactivation of Escherichia coli septation by the SOS and TER pathways. J. Bacteriol. 169: 1772-1776. 38. Drapeau, G.R., F. Gariepy, and M. Boule. 1984. Regulation and SOS induction of division inhibition in Escherichia coli K12. Mol. Gen. Genet. 193: 453-458. 39. Drapeau, G.R., J. P. Chausseau, and F. Gariepy. 1983. Unusual properties of a new division mutant of Escherichia coli. Can. J. Microbiol. 29: 694-699. 40. Dugaiczk A., H.W. Boyer, H.M. Goodman. 1975. Ligation of EcoRl endonuclease-generated DNA fragments into linear and circular structures. J. Mol.Biol. 96: 171-184. 41. Ferreira, L.C.S., W. Keck, A. Betzner, and U. Schwarz. 1987. In vivo cell division gene product interaction in Escherichia coli K-12. J. Bacteriol. 169: 5776-5781. 42. Friedman,D.I. and M. Gottesman. 1983. Lytic mode of lambda development, pp.21-51, In Lambda II (Hendrix, et al. eds.) Cold Spring Harbor, New York. 43. Fuller, R.S., B.E. Funnell, and A. Kornberg. 1984. The DnaA protein complex with the E. coli chromosomal replication origin (oriC) and other DNA sites. Cell 38: 889-900. 44. Gayda, R.C., and A. Markovitz. 1978. Cloned DNA fragment specifying major outer membrane protein a in Escherichia coli K-12. J. Bacteriol. 136: 369-380. 74 45. Gayda, R.C., L.T. Yamamoto and A. Markovitz. 1976. Second-site mutations in capR(lon) strains of Escherichia coli that prevent radiation sensitivity and allow bacteriophage lambda to lysogenize. J. Bacteriol. 127: 1208-1216. 46. George, J., M. Castellazzi and G. Buttin. 1975. Prophage induction and cell division in E. coli. III. Mutations sfiA and sfiB restore division in tif and Ion strains and permit the expression of mutator properties of tif. Mol. Gen. Genet. 140: 309-322. 47. Germino, J., J.G. Gray, H. Charbonneau, T. Vanaman, and D. Bastia. 1983. Use of gene fusions and protein-protein interaction in the isolation of a biologically active regulatory protein: the replication initiator protein of plasmid R6K. Proc. Natl. Acad. Sci. U.S.A. 80: 6848-6852. 48. Gill. D.R., G.F. Hatfull, and G.P.C. Salmond. 1986. A new cell division operon in Escherichia coli. Mol. Gen. Genet. 205: 134-145. 49. Grodberg, J. and J.J. Dunn. 1988. OmpT encodes the Escherichia coli outer membrane protease that cleaves T7 RNA polymerase during purification. J. Bacteriol. 170: 1245-1253. 50. Higgins, C.F., I.D. Hiles, G.P.C. Salmond, D.R. Gill, J.A. Downie, I.J. Evans, I.B. Holland, L. Gray, S.O. Buckel, A.W. Bell, and M.H. Hermodson. 1986. A family of related ATPbinding subunits coupled to many distinct biological processes in bacteria. Nature 323: 448-450. 51. Hirota, Y., F. Jacob, A. Ryter, G. Buttin, and T. Nakai. On the process of cellular division in E. coli. 1. Asymetrical cell division and production of DNA less bacterial. J. Mol. Biol. 35: 175. 52. Holland, I.B. 1987. Genetic analysis of the E. coli division clock. Cell 48: 361-362. 53. Holland, I.B. and C. Jones. 1985. The role of the FtsZ protein (SfiB) in UV-induced division inhibition and in the normal Escherichia coli cell division cycle. Ann. Inst. Pasteur Microbiol. 136A: 165-171. 54. Holmes, D.S., and M. Quigley. 1981. A rapid boiling method for the preparation of bacterial plasmids. Anal. Biochem. 114: 193-197. 55. Holtje, J-V, and U. Schwarz. 1985. Biosynthesis and growth of the murein sacculus. pp.77-120. In Molecular cytology of Escherichia coli (N. Nanninga,ed.), Academic Press. Ltd., London. 1968. 75 56. Huisman, 0., R. D'Ari. 1981. An inducible DNA replication-cell division coupling mechanism in E. coli. Nature(London) 290: 797-799. 57. Huisman, 0., R. D ’Ari and J. George. 1980. Inducible sfi dependent division inhibition in Escherichia coli. Mol. Gen. Genet. 177: 629. 58. Huisman, 0., R. D ’ari, and S. Gottesman. 1984. Cell-division and control in Escherichia coli: Specific induction of the SOS function SfiA protein is sufficient to block septation. Proc. Natl. Acad. Sci. 81: 4490-4494. 59. Ishidate, K., E.S. Creeger, J. Zrilce, S.Deb., B. Glauner, T.J. MacAlister, and L. I. Rothfield. 1986. Isolation of differentiated membrane domains from Escherichia coli and Salmonella typhimurium, including a fraction containing attachment sites between the inner and outer membranes and the murein skeleton of the cell envelope. J. Biol. Chem. 261: 428-443. 60. Ishino, F. and M. Matsuhashi. 1981. Peptidoglycan synthetic activities of highly purified penicillin-binding protein 3 in Escherichia coli: a septum-forming reaction sequence. Biochem. Biophys. Res. Commun. 101: 905-911. 61. Jaffe',A and R. D'Ari. 1985. Regulation of chromosome segregation in Escherichia coli. Ann. Microbiol. (Paris). 136A:159-164. 62. Jaffe', A., R. D'Ari, and V. Norris. 1986. SOS-independent coupling between DNA replication and cell division in Escherichia coli. J. Bacteriol. 765:66-71. 63. James, R., J.Y. Haga, and A.B. Pardee. 1975. Inhibition of an early event in the cell cycle of Escherichia coli by FL1060 an amidinopenicillanic acid. J. Bacteriol. 122: 1283-1292. 64. Jones, C.A., and I.B. Holland. 1984. Inactivation of essential division genes, ftsA, ftsZ suppresses mutations at sfiB, a locus mediating division inhibition during the SOS response in E. coli. EMB0 3: 1181-1186. 65. Jones, C.A., and I.B. Holland. 1985. Role of the sulb(ftsZ) protein in division inhibition during the SOS response in Escherichia coli: FtsZ stabilized the inhibitor SulA in maxicells. Proc. Natl. Acad. Sci. USA. 82: 6045-6049. 66. Jones, N.C., and W.D. Donachie. 1973. Chromosome replication, transcription and control of cell division in Escherichia coli. Nature(London) New Biol. 243: 100-103. 76 67. Kepes. F., and R. D'Ari. 1987. Involvement of FtsZ protein in shift up-induced division delay in Escherichia coli. J. Bacteriol. 169: 4038-4040. 68. Kren, B.E. and J.A. Fuchs. 1987. Characterization of the ftsb gene as an allele of the nrdB gene in Escherichia coli. J. Bacteriol. 169: 14-18. 69. Laemmli, U.K. 1970. Cleavage of structural proteins during the assembly of the head of bactriophage T4. Nature (London) 227: 680-685. 70. Lederberg, E.M. and S. N. Cohen. 1974. Transformation of Salmonella typhimurium by plasmid deoxyribonucleic acid. Bacteriol. 119: 1072-1074. J. 71. Little, J.W., and D.W. Mount. 1982. The SOS regulatory system of Escherichia coli. Cell 29: 11-22. 72. Lupski, J.R., A.H. Ruiz, and G.N. Godson. 1984. Promotion, termination and anti-termination in the rpsU-dnaG-rpoV macromolecular synthesis operon of E. coli K-12. J. Bacterol. 195: 391-401. 73. Lutkenhaus, J.F. 1983. Coupling of DNA replication and cell division: sulB is an allele of ftsZ. J. Bacteriol. 154: 1339— 1346. 74. Lutkenhaus, J.F. and H.C. Wu. 1980. Determination of transcriptional units and gene products from the ftsA region of Escherichia coli. J. Bacteriol. 143: 1281-1288. 75. Lutkenhaus, J.F., B. Sanjanwala, and M. Lowe. 1986. Overproduction of FtsZ suppress sensitivity of Ion mutants to division inhibition. J. Bacteriol. 166: 756-762. 76. Lutkenhaus, J.F., Donachie, W.D. 1979. Identification of the FtsA gene product. J. Bacteriol. 137: 1088-1094. 77. Lutkenhaus, J.F., H. Wolf-Watz, and W.D. Donachie. 1980. Organization of genes in the ftsA-envA region of the Escherichia coli genetic map and identification of a new fts locus (ftsZ). J. Bacteriol. 142: 615-620. 78. MacAlister. T.J., B. MacDonald, and L.I. Rothfield. 1983. The periseptal annulus: an organelle associated with cell division in Gram-negative bacteria. Proc. Natl. Acad. Sci. U.S.A. 80: 1372-1376. 79. MacAlister, T.J., W.R. Cook, R. Weigand, and L.I. Rothfield. 1987. Membrane-murein attachment at the leading edge of the division septum: a second membrane-murein structure associated with morphogenesis and the gram-negative bacterial division. J. Bacteriol. 169: 3945-3951. 77 80. Maguin, E., J. Lutkenhaus, and R. D'Ari. 1986. Reversibility of SOS associated divsion inhibition in Escherichia coli. J. Bacteriol. 166: 733-738. 81. Maniatis, T., E.F. Fritsch, and J. Sambrook. 1982. Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory, Cold Spring Harbor, New York. 82. Markiewicz, Z., J.K. Broome-Smith, U. Schwarz and B.G. Spratt. 1982. Spherical E. coli due to elevated levels of D-alanine carboxypeptidase. Nature(London) 297: 702-704. 83. Miller, J.H. 1972. Experiments in molecular genetics. Spring Harbor, New York. 84. Mirelman, D., Y. Yashouu-Gam, and U. Schwarz. 1977. Regulation of murein biosynthesis and septum formation in filamentous cell of Escherichia coli PAT84. J. Bacteriol. 129: 1593-1600. Cold 85. Mirelman,0., Y. Yashour-Gam, Y. Nuchamovitz, S. Rozenhak and E. Ron. 1978. Murein biosynthesis during a synchronous cell cycle of E. coli B. J. Bacteriol. 134: 459-461. 86. Mizusawa, S., D. Court, and S. Gottesman. 1983. Transcription of the sulA gene and repression by LexA. J. Mol. Biol. 171: 337— 343. 87. Nakamura, M., InN. Maruyama, M. Soma, J. Kato, H. Suzuki and Y. Hirrota. 1983. On the process of cellular division in Escherichia coli: Nucelotide sequence of the gene for penicillin binding proteins. Mol. Gen. Genet. 191: 1-9. 88. Nanninga, N., T. den Blaauwen, J. Voskuil, and F.B. Wientjes. 1985. Stimulation and inhibition of cell division in synchronized Escherichia coli. Ann. Inst. Pasteur(Paris) Microbiol. 136A:139-145. 89. Noel, G and G.R. Drapeau. 1986. Identification of new cell division genes in Escherichia coli by using extragenic suppressors. J. Bacteriol. 165: 399-404. 90. Oliver, D., and J. Beckwith. 1982. Identification of a new gene(secA) and gene product involved in the secretion of envelope proteins in Escherichia coli. J. Bacteriol. 150: 686-691. 91. Owen, P., P. Cafferey, and L.G. Josefsson. 1981. Identification and partial characterization of a novel bipartite protein antigen associated with the outer membrane of Escherichia coli. J. Bacteriol. 169: 3770-3777. 78 92. Raleigh, E.A. and N. Kleckner. 1986. Quantitation of insertion sequence IS10 transposase gene expression by a method generally applicable to any rarely expressed gene. Proc. Natl. Acad. Sci. U.S.A. 83: 1787-1791. 93. Reid, J.H., S. Patrick, E. Dermott, A. Trudgett, and S. Tabaquchali. 1985. Investigation of antigenic expression of Bacteroides fragilis by immunogold labelling and immunoblotting with a monoclonal antibody. FEMS Microbiol. Lett. 30: 289-293. 94. Richard, M., and Y. Hirota. 1973. Process of cellular division in Escherichia coli: physiolgical study on thermosensitive mutants defective in septum formation. J. Bacteriol. 116: 314-322. 95. Robinson, A.C., D.J. Kenan, G.F. Hatful1, N.F. Sullivan, R.S. Spiegelberg, and W.D. Donachie. 1984. DNA sequence and transcriptional organization of essential cell division genes /tsQ and ftsA. of Escherichia coli: Evidence for overlapping transcriptional units. J. Bacteriol. 160: 546-555. 96. Robinson, A.C., D.J. Kenan, J. Sweeny, and W.D. Donachie. 1986. Further evidence for overlapping transcriptional units in an Escherichia coli cell envelope-cell division gene cluster: DNA sequence and transcriptional organization of the ddl ftsQ, region. J. Bacteriol. 167: 809-817. 97. Rodriguez-Tebar, A., J.A. Barbas, and D. Vazquez. 1985. Location of some proteins involved in peptidoglycan synthesis and cell division in the inner and outer membranes of Escherichia coli. J. Bacteriol. 161: 243-248. 98. Rossen, L., C.A. Shearman, A.W.B. Johnston, and J.A. Downie. 1985. The presence of Rhizobium leguminosarum is autoregulatory and is the presence of plant exudate induced the nodA,B,C genes. EMBO J. 4: 3369-3373. 99. Sancar, A., A.M. Hack, and W.D. Rupp. 1979. Simple method for identification of plasmid-coded proteins. J. Bacteriol. 137: 692-693. 100. Sancar, A. R. P. Wharton, S. Seltzer, B.M. Kacinski, N.D. Clarke, and W.D. Rupp. 1981. Identification of the uvrk gene product. J. Mol. Biol. 148: 45-62. 101. Schoemaker, J.M., R.C. Gayda, and A. Markovitz. 1984. Regulation of cell division in Escherichia coli: SOS induction and cellular location of the SulA protein, a key to lonassociated filamentation and death. J. Bacteriol. 158: 551— 581. 79 102. Silhavy, T.J., M.L. Berman, and L.W. Enquist. 1984. Experiments with gene fusions. Cold Spring Harbor Larboratory, Cold Spring Harbor, N.Y. 103. Spratt, B.G. 1975. Distinct penicillin binding protein involved in the division elongation and shape of Escherichia coli K-12. Proc. Natl. Acad. Sci. U.S.A. 72: 2999-3003. 104. Spratt, B.G. 1977. Properties of the penicillin-binding proteins of Escherichia coli K-12. Eur. J. Biochem. 72: 352. 341— 105. Spratt, B.G., A. Boyd, and N. Stoken. 1980. Defective and plaque-forming lambda trasducing bacteriophage carrying penicillin binding protein-cell shape genes: genetic and physical mapping and identification of gene products from the Hp-dacA-rodA-pbpA-leuS region of the Escherichia coli chromosome. J. Bacteriol. 143: 569-581. 106. Spratt, B.G., and A.B. Pardee. 1975. Penicillin-binding proteins and cell shape in E. coli. Nature(London) 254: 515— 517. 107. Smith, M., and M.L. Birnstiel. 1976. A simple method for DNA restriction site mapping. Nucleic Acids Res. 3: 2389-2400. 108. Stoker, N.G., J.M. Pratt, and B.G. Spratt. 1983. Identification of the rodk gene product of Escherichia coli. J. Bacteriol. 155: 854-859. 109. Sullivan, N.F. and W.D. Donachie. 1984. Overlapping functional units in a cell division cluster in Escherichia coli. J. Bacteriol. 158: 1198-1201. 110. Sullivan, N.F., and W.D. Donachie. 1986. Transcriptional organization within an Escherichia coli cell division gene cluster: Direction of transcription of the cell separation gene envA. J. Bacteriol. 160: 726-732. 111. Tonak, M. and S. Hiraga. 1985. Negative control of oriC plasmid replication by transcription of the oriC region. Gen. Genet. 250: 21-26. Mol. 112. Taschner, P.E.M., J.G. J. Verest, and C.L. Woldringh. 1987. Genetic and morphological characterization of ftsft and nrdB mutants of Escherichia coli. J. Bacteriol. 169: 19-25. 113. Taschner, P.E.M., P.G. Huls, E. Pas, and C.L. Woldringh. 1988. Divsion behavior and shape changes in isogenic ftsZ, ftsQ, ftsA, pbpB and ftsE cell division mutants of Escherichia coli during temperature shift experiments. J. Bacteriol. 170: 1533-1540. 80 114. Teather, R.M., J.F. Collins, and W.D. Donachie. 1974. Quantal behavior of a diffusible factor which initiates septum formation at potential division sites in Escherichia coli. J. Bacteriol. 118: 407-413. 115. Tormo, A., A. Dopazo, A.G. de la Campa, M. Aldea, and M. Vincente. 1985. Coupling between DNA replication and cell division mediated by the FtsA protein in Escherichia coli: a pathway independent of the SOS response,the TER pathway. J. Bacteriol. 164: 950-953. 116. Tormo, A., and M. Vicente. 1984. The FtsA gene product participates in formation of the Escherichia coli septum structure. J. Bacteriol. 157: 779-784. 117. Tormo, A., C. Fermandez-Cabrera, and M. Vicente. 1985. The ftsk gene product: a possible connection between DNA replication and septation in Escherichia coli. J. Gen. Microbiol. 131: 239-244. 118. Tormo, A.,E.R. Martinez-Salas,and M. Vincente. 1980. Involvement of the ftsk gene product in late stages of the Escherichia coli cell cycle. J. Bacteriol. 141: 806-813. 119. Tormo, A., J.A. Ayala, M.A. de Pedro, M. Aldea, and M. Vincente. 1985. Interaction of FtsA and PBP-3 proteins in the Escherichia coli septum. J. Bacteriol. 166: 985-992. 12 0 . Utsumi,R., M. Kawamukai, H. Alba, M. Himeno, and T. Komane. 1986. Expression of the adenylate cyclase gene during cell elongation in Escherichia coli K-12. J. Bacteriol. 168: 14081414. 121. Ullmann, A. 1984. One-step purification of hybrid proteins which have B-galactosidase activity. Gene 29: 27-31. 122. Walker, J.R., Regulation mutants of formation. 123. Ward, J.E. and J. Lutkenhaus. 1984. A LacZ-FtsZ gene fusion is an analog of the cell division inhibitor SulA. J. Bacteriol. 157: 815-820. 124. Ward, J.E. and J. Lutkenhaus. 1985. Overproduction of FtsZ induces minicell formation in E. coli. Cell 42: 941-949. 125. Way, J.C., M.A. Davis, D. Morisato, D.E. Roberts, and N. Kleckner. 1984. New TnlO derivatives for transposon mutagenesis and for construction of LacZ operon fusions by transposition. Gene 32: 369-379. A. Kovarik, J.S. Allen, and R.A. Gustafson. 1975. of bacterial cell division: temperature-sensitive Escherichia coli, that are defective in septum J. Bacteriol. 123: 673-703. 81 126. Wientjes, F.B., T.J.M. Olijhoek, U. Schwarz, and N. Nanninga. 1983. Labeling pattern of major penicillin-binding proteins of Escherichia coli during the division cycle. J. Bacteriol. 153: 1287-1293. 127. Woldringh, C.L., P. Huls, N. Nanniga, E. Pas, P.E.M. Taschner, and F. B. Wientjes. 1988 Autoradiographic analysis of peptidoglycan synthesis in shape and cell division mutants of Escherichia coli MC4100. In Antibiotic Inhibition of Bacterial Cell Surface Assembly and Function. (P. Actor et al., eds) American Society of Microbiology, Washington D.C. 128. Wolf-Watz, H., and S. Normark. 1976. Evidence for a role of Nacetylmuramyl-L analine amidase in septum separation in Escherichia coli. J . Bacteriol. 128: 580-586. 129. Yi, Quing-Ming and J.F. Lutkenhaus. 1985. The nucleotide sequence of the essential cell division gene ftsZ of Escherichia coli. Gene 36: 241-247. 130. Yi, Q.-M, S. Rochenbach, J.E. Ward and J. Lutkenhaus. 1985. Structure and expresssion the the cell division genes ftsQ, ftsA, and ftsZ. J. Mol. Biol. 184: 399-412. Table 1. Strains Bacterial Strains Relevant markers Derivation,source or genotype RGC123 caRS(lon)tsul+ trp,lacAK74, capR9 (45) RGC103-9 capR9,sulBS (45) TKF10 ftsAlO(ts) (77) T0E1 ftsQ K. Beggs NK5012 supE,supF N. Kleckner CSR60 3 recAl,uvrA6,phr-1 B. Bachmann (CGSC #5830) PoII1734 mini-MudII1734 M. Casadaban (20) El 77 dnaA B. Bachmann AMC290 lacAX74 (23),TEY resistant,proC,trp AMC419 ftsZ84::TnlO A. Markovitz AMC421 ftsZ+::TnlO A. Markovitz AMC413 ftsl(ts)::TnlO(leu) A. Markovitz YC99 sulB9::TnlO This work YC100 ftsAlO::TnlO(leu) This work YC200 lac+ lac+ derivative of AMC290 Hfr3000 supQ80,relAl,spoT, B. Bachmann 82 Table 2. Plasmids and phages. Plasmids Relevant markers Source PRGC31 ampr, ftsZ+,ftsA+ R. Gayda(23) pRGC43 amp*",kan*",f tsA* ftsZ::mini-MuI1734(lac*) R. Gayda(23) pGW7 amp1" (30) PKK232-8 amp^ J. Brosius(16) pYCl6 kan^.ftsZ"" ftsA::mini-MuII1734(lac”) This work(23) V.anr ,ftsZ* ftsA::mini-MuIII734(lac*) This work(23) kan1",ftsZ* f tsA::mini-Mul117 34 (1aC") This work(23) kanr,ftsZ^ ftsA::mini-MulII734(lac*) This work(23) amp^.cm1amp^.cm1" ampr,cmr amp r,cm*" amp^.cm^ This This This This This pYC28 pYC39 pYC40 PYC500 PYC530 PYC580 pYC600 PYC620 work work work work work Phages PlVir X1098 (125) R. Gayda X::mini-TnlO N. Kleckner 84 Table 3. Cellular Location of FtsA and FtsA-LacZ Fusions Cellular Fractions cytoplasmic*___________ total membranes1 Sarkosy extracted fraction1 Plasmids FtsA P-gal fusion pRG43 .14 .05 pRG31 .13 FtsA .18 E-gal fusion <.01 FtsA .07 .12 E-gal fusion <.01 .06 pYC40 (142,000 fusion) .04 .05 .04 pYC28 (154,000 fusion) .03 .04 .03 1 - Relative molar yield of plasmid encoded proteins. Relative molar yield = % of polypeptide/molecular weight X 103 85 Table 4. Plasmids CAT/P-lactamase specific activities for different promoter gene fusions. Restriction frag. fused to Cmr gene Relevant Promoters PKK232-8 CAT/B-lactamase activity 0 PYC600 BamHl - ficoRl Pa.PzsPz 2 Pz 1 108 PYC620 Bglll - EcoiRl Pz3Pz2P*l 59 PYC580 Hindlll-EcoRl P2 2 PZ1 22 PYC530 Bglll-Hindlll PZ3 235 pYC500 Bam\-Hindlll Pa.Pz 3 249 Cell extracts of AMC290 containing above plasmids were prepared and CAT specific activity/0-lactamase activity was determined as described in Material and Methods. The background CAT activity in this assay was zero in AMC290(0DSOo = 0.5). All measurements were performed in duplicate and the mean is presented. 86 Table 5. Plasmids FtsZ effects on promoters. Restriction frag. fused to Cm*- gene CAT/fl-lactamase Relevant Promoters AMC419(ftsZ) AMC421(wt) time 30°C 4*°C 30°C 43°i (min) PYC500 PYC530 BarrMl-Hindlll Bglll-Hindlll P*P23 Z3 30 314 458 256 819 60 400 583 nd nd 30 164 377 244 930 60 179 507 nd nd nd = not done. A portion of actively growing cells (0D6Oo=0.1) were temperature shifted to 43°C and the cells were harvested after 30 and 60 minutes as described in Material and Methods. Table 6. Plasmids FtsQ effects on promoters. Restriction frag. fused to Cm*- gene Relevant Promoters CAT/P-lactamase activity X 103 TOEl(ftsQ) time 30°C 43°C (min) PYC500 PYC530 BaciHl-ifindlll Bglll-Hindlll P^PZ3 P« 0 334 30 369 957 60 325 1184 0 331 30 319 709 60 219 1158 Cell extracts were prepared and the activity was assayed as described in Table 5. 88 Table 7. Plasmids FtsA effects on promoters. Restriction frag. fused to Cm1- gene Relevant Promoters CAT/P-lactamase activity X 10s YClOO(ftsAlO) AMC290(wt) time 30°C 4*°C 30°C 43°C (min)______________________ PYC530 Bglll-Hindlll Pz3 30 234 208 54 94 PYC580 Hindll-EcoBl Pz2PZi 30 26 18 17 12 Cell were grown in L plus 0.2% glucose medium. Cell extracts were prepared and the activity was assayed as Table 5. Table 8. Plasmids DnaA46 mutants effects on promoters. Restriction frag, fused to Cmr gene Relevant Promoters CAT/8-lactamase activity X 103 E177(dnaAts) time 30°C 43°C (min) PYC500 pYC530 PYC580 BarMl-Hindlll Bglll-Hindlll Hindlll-EcoRl PaP z 3 ^23 PzaPzi 0 114 60 131 0 84 60 88 0 8 60 9 201 186 11 Strains culture was grown overnight in LB-0.2% glucose at 30°C and then diluted 100 fold and placed at 30°C. A portion of exponentially growing cells (0D6Oo = 0.5) was temperature shifted to 43°C for 60 min. The cells were collected and extracts prepared for assays as described in Materials and methods. 90 Fig. 1. Locations of coding sequences and promoters within the ddl- envA region of the E. coli chromosome, (a) The 14 loci concerned with cell envelope growth, organization and division have been identified within the 2-min region of the E. coli chromosome. enzyme map of structural gnes. transcription. the ddl-env.A region and, P, promoter locations; beneath, (b) Restriction location arrows, Abbreviations for restriction sites: of the directions of B, BamHl; Bg, Bglll; C, Clal; E, £coRl; H, Hindlll; K, Kpnl; P, PstI; Pv, PvuII; S, Sail; X, Xhol. m r aA m ra B B ESSPX ftsl tnurE m u r F tnurG m ur C ddl ftsQ ftsA ftsZ envA secA azi H F. B K rr 1L pv ddl f tsQ pw pw JT p* P* pi f tsZ f tsA 3 kilobases J I. i envA 1 92 Fig. 2. Proposed steps in the morphogenesis of E. coli cells. morphogenic systems are represented. of enzymes involed in the net Three System i: gene specifying a set synthesis of peptidoglycan(P.G.) including mraA, mraB, mrhA, mrbB, mrbC, mrcA, mrcB, murE, murf, murG, murC, ddl and possibly dacA and dacB. System ii: shape modifying system for the cylindrical P.G. sacculus. gene products assigned formation, for (those of ftsZ, ftsl, successive steps in completion, separation division sites) of new cell poles. ftsQ, cell and System iii: At least six ftsA, envA, division; inactivation the (as and minB) initiation, potential The phenotype of cells which have been blocked at different stages in system iii division process are shown at the top of the diagram. OCDC -> C D C D CZXZD ftsA, ftsl 1 mra A, B mrb A,B,C mrc A, B mur E,F,G,C ddl (dac A ,B) ~r (i) )Q envA minB 94 Fig. 3. Diagram of periseptal annuli in lkyD~ cells (a). Surface view of the chain of cells (b). Abbreviations: IM, inner membrane; M, murein; OM, outer membrane; P, plasmolysis bays; D, zones of membranemurein adhesion that form the periseptal annuli; D', zones of adhesion that form incomplete annuli at the mid points of the cells; S, septal cleft. (Modified from MacAlister et al (78)). 95 B 96 Fig. 4. line, Schematic representation of the expression vector pGW7. Thick DNA pBR322(30). derived from phage X; thin line, DNA derived from Arrows indicate the direction of transcription initiating from the X Pt. and X PR promoters. 97 Amp 98 Fig. 5. The vector pKK232-8. A partial restriction map shows the site in the polylinker used in promoter cloning. Amp;P-lactamase gene, T; terminators, CAT; chloramphenicol gene. Restriction enzyme codes: Ri, EcoRl; H3, Hindlll. 99 6 I 1---------- 1-----------1-----------1-----------f- kb Rl pvull Psti H3 R l Pvull 5.1 K b txr— ► 806066 terminators 100 Fig. 6. Restriction maps of pRGC31, Mudll 1734 and mini-Mu insertion locations. insertion Encoding sequence for ftsA showed as hatch line. sites are shown plasmid designations. by lollipop structure with Mini-Mu associated Restriction enzyme codes: Rl, EcoRl; pst, Pstl; Bl, BamHl; H3, Hindlll. Kanr is abbreviation for kanamycin resistance (25 abbreviation jig/ml). pg/ml). Ampr is for ampicillin resistance (50 1k t St H----------A E, — I------------------------- H M u d y 9 .7 Kb i* c Pst li E , ~l------- 1 ft S A K1734 Z' Kan c, 6, Amp' pRGC3 i 7.8 K D 101 102 Fig. 7. Autoradiograph of plasmid-specified polypeptides labelled in UV-irradiated maxicells. Polypeptides were separated on a 10% to 30% gradient SDS-PAGE. Lane A contained approximately 50% of the total radioactivity was that added to lanes B,C,D (500,000 c.p.m.). Molecular weight standards used were: myosin (205 kD), P-galactosidase (116 kD), phosphorylase B (97.4 kD), albumin, bovine (66 kD), albumin, egg(45 kD), carbonic anhydrase (29 kD). CSR603(pYC40); FtsA-LacZ Lanes: 1, CSR603(pRG31); 2, 3, CSR603(pYC28); 4, CSR603(pYC16). fusions, FtsA, FtsZ, protein specified by mini-Mu). Bla(P-latamase, Label point to Amp*") and Kan(Kanr 103 —CO O 0 QC Q. o CO CM CO _ O O o > >• > Q. Q. Q. 20511697- 66 ^ ‘- F t s A - L a c Z - 45- FtsA «-FtsZ ♦kV*. A B C D .104 Fig. 8. gradient. disrupted Separation of membrane fraction Strain AMC290(pYC40) and (Section XV). fractionated Areas as by sucrose equilibrium and AMC290(pRGC43) described corresponding to in materials 0MH , 0ML, F/B were and methods and described by Ishidate et al. (59) are indicated at top of Figure. IM as 105 OM OM F/ B 2. 0 - - - ■ 1.28 - - 1.24 - - 1.20 FtsA-LacZ 1.16 ■1.12 1 .0 - < La c Z 10 20 Fr a ct i o n Number 30 3 (g S u c r o s e / m l ) B-galactosidase units/ml 3.0 106 Fig. 9. pYC3. Schematic diagram for construction of expression plasmid, DNA segments of pYC16 (FtsA-LacZ plasmid) and vector pGW7 (X PL promoter plasmid regulated by thermolabile Cl repressor , Cxs57) were combined to generate pYC3. ftsA gene; PL, K PL [ promoter; ], MudII1734 DNA (9.7 Kb); m h h b , kanr, kanamycin resistance; amp^, ampicillin resistance; Z', B-galactosidase(truncated); N, X N gene. Restriction enzyme codes: Rl, £coRl; P, flstl; Bl, BamHl; H3, Hindlll\ Bg, Belli. 107 BH BH pCW7 8. 0K b 857 p Y C 16 17.5Kb f tsA Kan BH Amp Lac BH j d i g e s t i o n Bacterial and alkaline phosphatase treatment Partial digestion BHj purification of and 8.5Kb DNA Li ga t ion Selection BH Be 857 p YC3 1 6 . 5Kb Lac E Amp BH (Lac + , Amp r , Kan 5 ) with fragment. 108 Fig. 10. protein SDS-PAGE profile of proteins for purification of FtsA-LacZ from plasmid pYC3. temperature shifted AMC290(Alac) strain containing The cells were shifted to 42°C for 2.5 hrs. The hybrid protein was purifed from the soluble fraction of French pressure lysed cells by ammonium sulfate precipitation and affinity chromatography on p-aminobenzyl-0-D-thio-galactopyranoside in the Material and Methods. agarose(Sigma) as described Polypeptides were separated on a 10 to 30% gradient gel and stained with Coomassie blue (0.2% w/v). A. molecular wight standards( same as described in Fig. 8); protein in broken cells (140 yg); (130 yg); D.40% Lanes: B. total C. total protein in cleared lysate (Nm)2S0A pellet(130 yg); E. purified FtsA-LacZ fusion protein eluted from p-aminobenzyl-B-D-thio-galactoside column (10 yg). 109 F tsA -L acZ ** LacZ 110 Fig. 11. protein Identification of FtsA by Western blot analysis. was [35S]methionine labelled specifying FtsA and/or P-galactosidase. min at 100°C in mercaptoethanol. sample buffer Proteins were electro-transferred onto Nytran in maxicells with FtsA plasmids Each sample was heated for 5 containing 4M urea separated by sheets. Blocked and 1% SDS-PAGE(10-30%) membranes Pand were incubated with purified IgG antibodies (1:1000 dilution) against FtsALacZ for 2 hrs and washed. Immunogenicity was detected with alkaline phosphatase linked goat-anti-rabbit-IgG with color development by BCIP and NBT. Maxicell labelled polypeptides were detected in the same blot by autoradiography. A). Autoradiogram of blot B. CSR603/pRGC43; lane 2 - CSR603/pRGC31. of FtsA in total cells (blot B). CSR603/pRGC31. Lanel - CSR603/pRGC43; lane 2 Lane 1 - CSR603/pRGC31; (1/5 cpm); lane3 - CSR603/pYC40 fusion); lane 4 - CSR603/pYC16 (FtsA-LacZ fusion). detection of FtsA or FtsA-LacZ fusion (blotD). pRGC31 specifies FtsA, FtsZ, Amp. FtsA, FtsZ, Amp, Kan. - B). Immunoblotting detection C). Autoradiogram of blot D. lane 2 - CSR603/pRGC43 Lanel (FtsA-LacZ D). Immunoblotting lanes 1-4 same as C. pRGC43 specifies 3-galactosidase, Ill Fts A-Lac FtsA FtsA 112 Fig. 12. Immunodetection of FtsA in AMC290 strain harboring pYC40 or overproducing performed 12. plasmid pYC3. Western blotting experiments were under same conditions as described in the legend of Fig. Lanes: A. 40% (NHA)2SOA precipitate of AMC290/pYC40 extract (150 yg); B. 40% (NHa )2S0* precipitate of temperature induce AMC290/pYC3 extract (65 yg). 113 A B 114 Fig. 13. Cellular localization of the FtsA protein by Western blot with FtsA antibodies. Membrane fractions of AMC290 were isolated by the procedure of Ishidate et al. (59) as described in Fig. 9. sample(~50yg) was heated containing 4M urea and for 20 min at 90°C 1% P-mercaptoethanol Western blot analysis. in sample Each buffer prior to SDS-PAGE and The Western blot was done under the same experimental conditions described in Fig. 12. Lanes: 1. IM - inner membrane; 2. F/B - Flagellar fraction with membrane fragments; 3. - outer membrane light fraction; 4. 0MH - outer membrane 0ML heavy fraction;5. Total membrane; 6. Total soluble proteins; 7. Control- 40% (NHa )2S0* of temperature induced membrane; 9. Total soluble proteins. AMC290/pYC3 extract; 8. Total U5 "'S 1 2 3 4 5 6 7 8 9 116 Fig. 14. Western blot with FtsA-LacZ antibodies of cell fractions of AMC290. Approximately equal amount fractions of AMC290 conditions were were applied as described (~50yg) protein from cellular to in Fig. each 14. lane. Lanes: Experimental A. IM - inner membrane; B. F/B - flagellar fraction with membrane fragments; C. OMt, - outer membrane fraction; control E. - light fraction; D. total membrane pellet; CSR603/pRGC31 total cell 0M„ F. - outer membrane heavy total soluble proteins; proteins; H. (NH^)2S0* of temperature induced AMC290/pYC3 exract. control - G. 40% 117 *K**tn. *-A*fl% A B C D E F G H FtsA 118 Fig. 15. SDS-PAGE Coomassie blue stain gel profile of AMC290 cellular fractions analysed in immunoblot of Fig. 15. and separated as descibed in Fig. 15. Each sample was prepared Lanes: A. IM - inner membrane; B. F/B - flagellar fraction with membrane fragments; C. OMr. - outer membrane light fraction; D. 0M„ - outer membrane heavy fraction; E. total membrane CSR603/pRGC31 pellet; total F. total cell proteins; soluble H. temperature induced AMC290/pYC3 exract. proteins; control - 40% G. control - (NHA)2S0* of 119 | I g _ J ||| 66 - mum.- m*ms* 45 - 29- Vjfl 1 2 3 4 5 6 7 8 9 120 Fig. 16. Western blot of FtsA antibodies membrane of cell fractions of AMC290. preabsored with total Approximately equal volume of each sample was prepared and analyzed as described in Fig. 15. FtsA- LacZ specific antibody faction was preabsorbed twice with lysate of ftsAlO mutant containing the total membrane fractions isolated from a sucrose step gradient. Lanes: 1. IM - inner membrane(60 yg); 2. F/B flagellar fraction with membrane membrane light fraction(60 yg); fragments(10 yg); 4. 0M„ - outer 3. 0ML - outer membrane heavy fraction(40 yg); 5. total membrane pellet(50 yg); 6. total soluble proteins(50 yg); 7. control - CSR603/pRGC31 total cell proteins; 8. control - 40% (NHa)2SOa of temperature induced AMC290/pYC3 exract(50 yg). 121 'r. v:"^ /i- • . i Ft s A : 8111# ?■••• 'l\ .■;.; '■=M 2 3 ~ 4 5 6 7 8 122 Fig. 17. Electron microscopic localization of FtsA in E. coli cells by immunogold labelling. Cells were embedded, thin sectioned, treated with FtsA-LacZ polyclonal rabbit IgG colloidal Material and Methods. antibodies gold and labelled with goat conjugates(lOnm) as described Lanes: A,B,C,D - AMC290(Alac) in anti the treated with FtsA-LacZ polyclonal antibodies; E,F - control, AMC290 treated with pre-immune serum antibodies; G,H - control, YC200(lac*) treated wtih FtsA-LacZ polyclonal antibodies. 123 •Mt ,»■ r \y&uF^ ^0T w ••' »r.. . 124 125 Fig. 18. Phase contrast light and electron micrographs of plasmolyzed ftsZ84(ts) filaments. Strain AMC419 growing cells at 30°C (0DfeOo = 0.2) in YET broth were shifted to 42°C for 90 min. A portion of the culture was plasmolyzed in 13% sucrose, fixed with paraformaldehyde and prepared for phase contrast (A) and electron microscopy(B). Plasmolysis bays are visible along the lenght of the filaments. pm - light micrograph; Bar, ! pm - electron micrograph. Bar, 126 A 127 Fig. 19. Immunoelectron micrographic localization of FtsA in ftsZ8A filament. A portion paraformaldehyde and of culture prepared described in the legend to Fig. 18. in for Bar, Fig. 18 was fixed immuno-electromicroscopy | ym. with as 128 129 Fig. 20. Localization of FtsA in plasmolyzed ftsZ84 filament by immunoelectron micrograph. This section of plasmolyzed ftsZ filamentous cells in Fig. 18 was subject ot immunogold labelling as described in Fig. 18. } pm. Bar 130 131 Fig. 21. Promoter fusions of the ftsA-ftsZ regulatory region to the chloramphenicol acetylase gene(Cm*"). A partial restriction map of the 2.2 Kb fragment from pRGC31(Fig.6 ) is shown together with the extent of cloned fragments and their orientation relative to Cm*" structural gene in pKK232-8. and the structural Also depicted are the promoter (Pzl, Pz2, Pz3, PA ) regions of ftsQ, incomplete coding region). ftsA, ftsZ. (" ' " indicated Abbreviation used for restriction enzymes are Ei, EcoRl; Bi, BamHl; Bg, Bglll; Bs, BssHI; H3, Hindlll. *t»Q' UtZ‘ «HA BomHi BoMi fc= r- Hindiu -i EcoRl 1 i,l - > — PA — > PZ3 » PZ21 ^ H3 pYC 500 Z3 Bg pYC 530 ■>H: Z3 H, pYC 580 Z21 r > El PZ3 PZ2l B g ----------P ^ Z3 pYC 600 P Z21 pYC 620 VITA Younghae Chong Chon was born August 4, 1953 in Seoul, Korea. She graduated from Jeongshin Girls High School in Seoul, Korea in 1972. She attended Ewha Women's University received a Bachelor of Pharmacy degree in 1976. worked for more Investigation in than 3 years at National Seoul, Korea in a in Seoul, Korea and After graduation she Institute of Scientific laboratory as a pharmacist. Younghae entered the Graduate School of Louisiana State University in August 1982 and received the Master of Science degree in Microbiology in December 1984. She is currently a candidate for the degree of Doctor of Philosophy in Microbiology at Louisiana State University. 133 DOCTORAL EXAMINATION AND DISSERTATION REPORT Candidate: Major Field: Younghae Chong Chon Microbiology Title of Dissertation: Cell Division Studies of Escherichia c o li ; Expression and Protein Localization of a Cell Septation Gene, ftsA. Approved: Major Professor and Chairman Dean of the GraduatfKschooI EXAMINING COMMITTEE: Date of Examination: Tuesday, July 12, 1988