* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Chemical reaction
Molecular orbital diagram wikipedia , lookup
Radical (chemistry) wikipedia , lookup
Protein adsorption wikipedia , lookup
Nucleophilic acyl substitution wikipedia , lookup
History of molecular biology wikipedia , lookup
Metastable inner-shell molecular state wikipedia , lookup
Click chemistry wikipedia , lookup
Stoichiometry wikipedia , lookup
Chemistry: A Volatile History wikipedia , lookup
Organic chemistry wikipedia , lookup
History of chemistry wikipedia , lookup
Marcus theory wikipedia , lookup
Metallic bonding wikipedia , lookup
Electron configuration wikipedia , lookup
Hydrogen bond wikipedia , lookup
Chemical thermodynamics wikipedia , lookup
Water splitting wikipedia , lookup
Isotopic labeling wikipedia , lookup
Transition state theory wikipedia , lookup
Chemical reaction wikipedia , lookup
Hydrogen-bond catalysis wikipedia , lookup
IUPAC nomenclature of inorganic chemistry 2005 wikipedia , lookup
Electrochemistry wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Lewis acid catalysis wikipedia , lookup
Rutherford backscattering spectrometry wikipedia , lookup
Nucleic acid analogue wikipedia , lookup
Molecular dynamics wikipedia , lookup
Artificial photosynthesis wikipedia , lookup
Resonance (chemistry) wikipedia , lookup
Physical organic chemistry wikipedia , lookup
Acid–base reaction wikipedia , lookup
Electrolysis of water wikipedia , lookup
Photosynthetic reaction centre wikipedia , lookup
Fatty acid metabolism wikipedia , lookup
Hypervalent molecule wikipedia , lookup
Abiogenesis wikipedia , lookup
Chemical bond wikipedia , lookup
Metalloprotein wikipedia , lookup
Atomic theory wikipedia , lookup
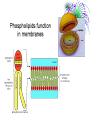
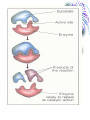
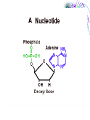
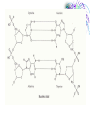
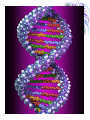
Chemistry & Biochemistry Chapters 2 & 3 Atoms • Matter - anything that occupies space and has mass • Three states • Solid • Liquid • Gas • Atoms - the smallest units of matter that have their own distinct properties Atomic Structure • Nucleus • Protons (+) • Neutrons (0) • Electron Shell • electrons (-) Chemical Element • Chemical elements - composed of atoms that share the same characteristics, but differ from the atoms of other elements • Elements want eight electrons in the outermost shell • Except Hydrogen and Helium Helium Compounds • A pure substance made up of atoms of 2 or more elements • A molecule is the simplest part of a substance that retains all of the properties of the substance Water Elements • • • • • • • • • Oxygen - O Carbon - C Hydrogen - H Nitrogen - N Calcium - Ca Phosphorus - P Sulfur - S Sodium – Na Potassium - K • • • • • • • • • Magnesium - Mg Chlorine - Cl Iron - Fe Iodine - I Manganese - Mn Copper - Cu Zinc - Zn Cobalt - Co Fluorine - F Ionic Bonds • A positive ion and negative ions held together • Electrostatic - forces of attraction • A gain or loss of electrons • Cations - positively charged ions • Anions - negatively charged ions Covalent Bond • Sharing electrons with other atom • H-H • O=O Hydrogen Bonds • Weak bonds between hydrogen and a weak negative charge • In large numbers they provide strength to molecules • Important in DNA Basic Terms • Chemical reaction: • Reactant: a compound or atom involved in a chemical reaction • Product: a compound formed by a chemical reaction Reactions • In a reaction, bonds present in reactants are broken, elements are rearranged, and new compounds are formed as products. • Example: C12H22O11 +H2OC6H12O6 + C6H12O6 Synthesis Reactions • A + B AB • Requires the formation of new bonds between the combining units (reactants) • Important in repairing worn or damaged parts Examples Decomposition Reactions • AB A + B • Breaking down of molecules into simpler molecules • Digestion Example Transfer of Energy Reactions • Exergonic reactions: net release of free energy • Endergonic reactions: net absorption of free energy Energy Transfer • Activation Energy - the amount of energy needed to start a chemical reaction • Catalysts - reduce the amount of energy needed Enzymes: important class of catalysts in living things • EX. Oxidation-Reduction Reactions • Transfer of energy by the transfer of electrons • Oxidation reactions lose an electron • Reduction reactions gain an electron Redox Reactions Memory TIP •OIL RIG •“OIL” = oxidation loses electrons •“RIG” = reduction gains electrons Terms • Solution – a mixture in which one or more substances are uniformly distributed into another • Solute – the substance dissolved in the solution (Sugar) • Solvent – the substance in which the solute is dissolved (Water)[aqueous] Concentration • Concentration: the amount of solute dissolved in a fixed amount of solution •Saturated solution: no more solute can dissolve Concentration Cont. • % Concentration solute = • Mass of solute/ mass of solution X 100% • % Concentration solvent= • Mass of solvent/ mass of solution X 100% Kool-Aid • What is the percent concentration of sugar? • 10 ml water • 5g Sugar • 2g Kool-Aid Mix Acids and Bases • Neutral= number of hydronium ions equal the number of hydroxide ions • Acid =number of hydronium ions in a solution is greater than the number of hydroxide ions (H) • Base= number of hydroxide ions in a solution is greater than the number of hydronium ions (OH) pH Scale • • • • 0 to 14 0 = very acidic 14 = very basic 7 = neutral Acid Vs. Base • Release H+ • Sour • Corrosive • Red (pH indicators) • • • • • Release OH – Bitter Alkaline Feels Slippery Blue (pH indicators) Buffers • Buffers: chemical substances that neutralize small amounts of either an acid or a base when added to a solution • Carbonic acid/ bicarbonate • Keep blood at a pH of 7.4 Water • Most abundant compound in living things • 60-70% of total body mass • Important transport medium • High heat capacity - absorbs and releases heat slowly • Effective lubricant Water’s Polarity • Polar – unevenly pattern of charges • able to dissolve many substances • Positive on one end and negative on the other. Polar Molecules Dissociation of Water • The breaking apart of water molecules into two ions of opposite charge H2O H+ + OHH+ + H2O H3O+ OH- = hydroxide ion H3O+ = hydronium ion Cohesion • Cohesion: attractive force between particles of the same kind Example: surface tension of water • Polar nature of water causes water molecules to be attracted to each other. • This attraction: hydrogen bond. Adhesion • Adhesion: attractive forces between unlike substances Basic Info. • Very large organic molecules composed of many smaller molecules • All organisms composed of 4 major classes of macromolecules: Carbohydrates Proteins Lipids Nucleic acids Carbon • Primary component of all macromolecules • Atom has 4 electrons in the outer energy level. • Readily forms 4 covalent bonds with other elements Forms straight chains, branched chains, or rings Organic (Carbon) Compounds • Contain carbon atoms that are covalently bonded to other carbon atoms and to other elements : Typically hydrogen (H), nitrogen (N) and oxygen (O) Macro( Large) Molecules • Macromolecules are built from smaller simpler molecules called monomers. • Monomers bond to one another to form complex molecules called polymers Monomers to Polymers Carbohydrates • • • • • sugars & starches energy source Monosaccharide - simple sugar (glucose) `(“Mono-” means “one”) Disaccharide - table sugar (“Di-” means “two”) • Polysaccharide - lack sweetness (“Poly-” means “many”) Monosaccharide Isomers • Isomers- have the same chemical formula, but have different properties because of their structure. Dissacharides • Formed when 2 monosaccharides combine by a condensation reaction or dehydration synthesis. • Double sugars • Example: glucose + fructose sucrose + water Example Polysaccharides • Complex molecules composed of 3 or more monosaccharides. • Insoluble in water. • Examples • Glycogen -synthesized by animals • Cellulose -found in the cell walls of plants • Starch Examples Lipids • Major roles in living organisms are: Store energy Form biological membranes Chemical messengers Insulation Lipids • Do not dissolve in water Examples • Steroids • Testosterone and Cholesterol • Waxes • Plants and animals Fatty Acids • Most lipids are made of fatty acids. • Unbranched carbon chains Fatty Acids Cont. • Two ends of fatty acid: Carboxyl (COOH) group Hydrocarbon (long chains of hydrogen and carbon atoms) Saturated Vs Unsaturated Fats • Each carbon is covalently bonded to 4 atoms. • Contains the maximum number of hydrogen atoms. • The atom is full or “saturated”. • Each carbon atom is not bonded to 4 atoms. • Does not contain the maximum number of hydrogen atoms. • One or more carbon atoms are double bonded to each other. Complex Lipids • Divided according to structure. • Three classes of lipids important to living things contain fatty acids: Triglycerides Phospholipids Wax Triglycerides • 3 molecules of fatty acids joined to one molecule of glycerol. Saturated Vs Unsaturated Triglycerides High melting point. Solid at room temp. Examples: shortening and animal fats. Usually liquid at room temp. Found primarily in plant seeds and fruits. Phospholipids • Have 2 rather than 3 fatty acids joined to a molecule of glycerol. • Cell membranes composed of 2 layers of phospholipids: lipid bilayer. Produces a stable barrier for a cell. Phospholipids cont. Wax • Type of structural lipid. • Highly waterproof. In plants, form protective outer coating. Also forms protective layers in animals. Cholesterol Plant Wax Little Ear Wax Lots of Wax Steroids Not composed of fatty acids. Sex hormones Cholesterol Chlorophyll (pigment in plants) Retinol (vision pigment in eyes) Proteins • Composed of long chains of subunits (monomers) called amino acids. • Covalent bond between 2 amino acids form peptide bonds • More than 50 = protein Amino Acid Structure • Each contain a central carbon atom covalently bonded to 4 other atoms or functional groups: A single H atom at one site. A carboxyl group (-COOH) bonds at a second site. Cont. An amino group (-NH2) bonds at a third site. A functional group (“R”) bonds at the fourth site. R (functional group) NH2 C (amino group) H COOH (carboxyl group) 20 Different Kinds of Amino Acids • Main difference among amino acids is found in their “R” (functional) group. Dipeptides • Two amino acids can bond to form a dipeptide. • Condensation reaction • Creates water • Forms a peptide bond Peptide Bond Polypeptide • 3 or more peptides bind together to make a polypeptide chain. • Some are very chains are so long, they are bent or folded. • Temperature can determine the shape. Enzymes • Most enzymes are proteins. • Enzyme reactions depend on a physical fit between the enzyme and its substrate (the reactant being catalyzed). Enzyme and Substrate How Enzymes Work • Reduce activation energy. Enzyme is unchanged. May be used many times. • May fail to work if environment is changed. Temperature or pH can cause a change in the shape of the enzyme or substrate. Nucleic Acids • Store important hereditary information in the cell. • 2 types: DNA and RNA DNA/RNA Basic Info • Both DNA and RNA are polymers. Composed of 1000’s of linked monomers called nucleotides. • made of 3 components: Phosphate group 5 carbon sugar Nitrogen base Deoxyribonucleic acid • DNA • Double helix • Contain • nucleotides • a deoxyribose sugar • nitrogen bases (thymine, adenine, cytosine, guanine) • phosphate group DNA Ribonucleic acid • RNA • Stores and transfers information essential for the manufacture of proteins. RNA More terms to know • Free energy • ATP • Hydrolysis