* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download UNIT 2: BIOCHEMISTRY/ENZYMES
Interactome wikipedia , lookup
Isotopic labeling wikipedia , lookup
Western blot wikipedia , lookup
Two-hybrid screening wikipedia , lookup
Genetic code wikipedia , lookup
Photosynthetic reaction centre wikipedia , lookup
Amino acid synthesis wikipedia , lookup
Nucleic acid analogue wikipedia , lookup
Protein–protein interaction wikipedia , lookup
Biosynthesis wikipedia , lookup
Nuclear magnetic resonance spectroscopy of proteins wikipedia , lookup
Metalloprotein wikipedia , lookup
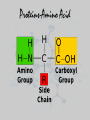
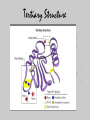
UNIT 3: BIOCHEMISTRY/ENZYMES Miss Sabia 8A Essential Question How do organic and inorganic compounds compare? First things first… • Element: a pure substance that consists entirely of one type of atom • Compound: chemical substance formed by the combination of 2 or more elements in definite proportions • For example, H2O is made of 2 hydrogen atoms and 1 oxygen atom First things first • A chemical formula tells us the type of elements that are in a compound and the ratio in which those atoms combine • Glucose, C6H12O6 Some quality bonding time… • Hydrogen Bonds: weak bonds of attraction between the partially charged H atom and another partially charged atom – Between water molecules Some quality bonding time… Ionic Bonds Covalent Bonds • Electron transfer due to electrical attraction between ions • Form cations (+) and anions (-) • Electron sharing • Can be polar (unequal sharing) or nonpolar (equal sharing) Electronegativity: “greediness” for electrons; attraction of an atom for electrons in a covalent bond Organic Compounds • CONTAIN CARBON!! • Most also contain hydrogen • Associated with living things Why is carbon so special? • Think of carbon as the jack-of-all-trades • Has potential to form many kinds and combinations of bonds with many different atoms—able to form 4 covalent bonds Essential Question What are the four classes of organic molecules? The Macromolecules • • • • Carbohydrates Nucleic Acids Proteins Lipids Carbs Lipids Nucleic Acids Nucleic Acid Proteins-Amino Acid Protein Activity • You will make a placemat about the 4 types of carbon molecules. Include: • 1. a picture of a food that contains each type of molecule (you may not be able to find one for nucleic acids, which is fine). • 2. For each molecule, include a description, as well as a drawing of what the actual carbon molecule looks like. • 3. Your placemats will be laminated and ready for you to use! Do Now • Name as many functions of a protein as you can… Essential Question •How is a protein’s function determined? How do we get these macromolecules? • When we eat, large organic food molecules such as proteins and starches must initially be broken down to enter cells • Proteins amino acids • Starches simple sugars • These nutrients can now enter the cell and be used as building blocks of compounds needed for life Vocabulary • Monomer: single unit • Polymer: many monomers Reactions • Dehydration synthesis: joining molecules together, results in loss of water • Hydrolysis: breakdown of polymers through the addition of water A closer look at Proteins • SHAPE DETERMINES FUNCTION!!!!!! • 4 levels of protein structure – 1. primary – 2. secondary – 3. tertiary – 4. quaternary Primary Structure • Amino acid sequence Secondary Struture • Coiling or folding of the a.a. sequence due to hydrogen bonds Tertiary Structure • Irregular contortions from interactions between side chains (aka R groups) • This involves… – Hydrogen bonding – Ionic bonding – Hydrophobic interactions Tertiary Structure Quaternary Structure • Grouping of polypeptide chains SHAPE DETERMINES FUNCTION INSULIN How is the structure determined? • Structure depends on the environment – pH – Temp – Salt [] Denaturation • A poor environment may result in denaturation (breaking of a protein) – Cooking an egg – Getting a fever It all comes down to amino acids • What do you think happens if you change the sequence of amino acids? Essential Question • What is the role of enzymes in a chemical reaction? Essential Question • What factors affect the rate of enzymatic reactions?