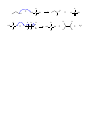* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Organic Reactions
George S. Hammond wikipedia , lookup
Cracking (chemistry) wikipedia , lookup
Physical organic chemistry wikipedia , lookup
Asymmetric induction wikipedia , lookup
Tiffeneau–Demjanov rearrangement wikipedia , lookup
Wolff–Kishner reduction wikipedia , lookup
Baylis–Hillman reaction wikipedia , lookup
Ring-closing metathesis wikipedia , lookup
Wolff rearrangement wikipedia , lookup
Ene reaction wikipedia , lookup
Petasis reaction wikipedia , lookup
Hydroformylation wikipedia , lookup
Organic Reactions 1. 3 basic kinds of reactions – similar to those from Chem 1 but called something else a. Addition Reactions (like synthesis reactions) i. Hydrogenation 1. saturating an unsaturated carbon chain 2. alkene/yne to alkane ii. Hydration 1. alkene to alcohol iii. Halogenation/Hydrohalogenation 1. alkane to haloalkane 2. alkene to haloalakane b. Elimination Reactions (like synthesis reactions because they result in larger compounds) i. Condensation 1. Esterification 2. Formation of alkene 3. Formation of amide (peptide bond) c. Substitution Reactions (like single or double replacement reactions where one atom/ion/functional group is replaced by another) i. SN1 ii. SN2 2. Basic process of organic reactions is through attraction of positively and negatively charged parts of molecules a. Organic has special names for positively and negatively charged parts of a molecule Electrophiles “loves electrons” = attracted to negative charge maybe positively charged or have deficit of electrons b/c atom is attached to very electronegative atom carbon of carbonyl group acids Nucleophiles “loves nuclei” = attracted to positive charge often negatively charged or lone pairs high electronegativity alkenes Hydroxide –OH Chloride –Cl Ammonia – NH3 b. many organic reactions happen through the attraction of electrophiles for nucleophiles c. in reaction mechanisms, curly arrows show how electrons move – generally electrons from nucleophile move to electrophile 3. Alkanes are relatively inert compared to other functional groups a. Alkenes have pi bonds in which electrons are easily accessible b/c they aren’t trapped between two nuclei as sigma bonding electrons are. b. Other functional groups have highly electronegative atoms like O, N or halogens 4. The table below gives the characteristic reactions for several functional groups a. The name of the rxn is given followed by what it makes in ( ) Functional Group Addition Elimination Substitution Alkane Halogenation (haloalkanes) Alkene Hydrohalogenation (monohaloalkanes,) Hydration (alcohols) Halogenation (dihaloalkanes) Hydrogenation (alkanes) Oxidation (-OH, C=O, COOH) Alcohol Condensation Oxidation (aldehyde, w/ COOH to (ester) ketone, COOH) w/ conc. acid or catalyst (alkene) Carboxylic Acid Condensation with –OH (ester) Amine Condensation w/ COOH (amide) Reactions 1. Halogenation of alkane a. Alkane + halogen gas haloalkane b. Need ultraviolet light for rxn to occur c. Depending on time and amount of reactants, more than one halogen can added to the alkane d. Also see Nucleophilic Substitution Notes H H H H Cl h h h h H H + Cl Cl Cl H Cl Cl Cl Cl Cl Cl H H H Cl Cl methane chloromethane dichloromethane chloroform also: trichloromethane tetrachloromethane also: carbontetrachloride e. Reaction occurs through homolytic fission to form a free radical (HL only) i. Free radical is a element or molecule with an unpaired electron ii. Homolytic fission vs Heterolytic fission: 1. Fission means splitting apart 2. Homolytic means the bond is split in half – each side takes 1 electron and 2 free radicals are formed 3. Heterolytic means that one atom takes both electrons in the bond and two ions are formed. iii. Formation of free radicals often results in chain reactions – reaction keeps occurring until all reactant is used up. See polymerization notes form mechanism. 2. Hydrohalogenation a. Alkene + acid halide monohaloalkane b. Halide ion adds to larger side (more substituted side of alkene) i. Hydrohalogenation of ethene H H H H + H Cl H Cl H H H H ii. Hydrohalogenation of 1-propene: notice that the chlorine adds to the larger side of the alkene. H H H H + H H H CH3 c. Reaction occurs through heterolytic fission to form an ion (HL only) i. The first step is the attraction of the electrophile (Hydrogen ion) to the electrons in the pi bond. This forms a carbocation. ii. The carbocation that is more substituted (has more carbons attached to it) is the most stable. iii. The negatively charged halogen (nucleophile) adds to the carbocation to form the halogenated alkene. H H CH 3 CH 3 CH 3 + H CH3 H Cl H Cl H H Cl C H H + + Cl- H H H 3. Hydration a. Alkene + water in acidic solution alcohol b. Acid acts as catalyst in rxn c. –OH group adds to larger side (more substituted side) of alkene d. Uses: hydration is used for commercial manufacture of ethanol i. Hydration of ethene H H H H H H acid + H O H H O H H ii. Hydration of 1-propene H H H CH3 acid + H O H H H H H O H H CH3 H Cl 4. Halogenation a. Alkene + halogen gas 1,2-dihaloalkane b. Diatomic gas has two atoms – both add to opposite sides of the double bond (and opposite sides of the molecule) c. Uses: Chlorine + ethane 1,2-dichloroethane: used as starting material for PVC d. Uses: Br2 dissolved in dichloromethane is used to distinguish between alkenes and alkanes. If reddish-brown color of Br2 disappears when added to unknown, the unknown has alkenes in it. H H H H + Cl Cl Cl Cl H H H H 5. Hydrogenation a. Alkene + Hydrogen gas (with catalyst) alkane b. Hydrogenation is saturating an unsaturated hydrocarbon c. Also called reduction (carbon is reduced in this reaction but is also reduced in many of the reactions above) d. Heterogeneous Catalyst: Pd or PtO2 (rxn occurs on a metal surface) e. Uses: unsaturated vegetable oils are saturated to produce saturated fats (more solid at room temp than unsaturated) for margarines H H H H PtO2/Pd + H H H H H H H H 6. Esterification a. Carboxylic acid + alcohol ester + water b. Reaction conditions: acidic solution c. The OH group on the carboxylic acid is replaced by the alcohols O-R group d. Condensation reaction: produces water e. Uses: flavouring agents, plasticizers, as solvents in perfume, polyesters O OH acid + propan-1-ol H2O ethyl acetate OH + acid O + H2O O O propan-1-ol propyl propionate propionic acid f. + O acetic acid OH O OH Mechanism: O H + R O H Cl + OH R OH R HO OH OH R O + H O H + R H OH R O + H H O + H H + O H R O OH O R R + + H 7. Amide formation a. Carboxylic acid + amine amide + water b. Reaction condition: difficult to conduct in simple steps since amine (a base) and acid basically neutralize each other. To form amide, other reactions that “protect” important functional groups are required. c. The OH group on the carboxylic acid is replaced by the amine (NH-R) d. Condensation rxn: produces water e. Uses: peptide bond formation, polymerization reactions to make nylons O H3 C + OH acetic acid also: ethanoic acid O H2N CH3 methanamine H3 C NH CH3 N-methylacetamide O + OH butyric acid also: butanoic acid f. CH3 O H CH3 N CH3 N-methylmethanamine N,N-dimethylbutanamide Reaction mechanism O C O CH3 HN CH3 + O CH3 N H CH3 CH3 O- C + H + N CH3 CH3 H2O CH3 O CH3 C N CH3 H 8. Oxidation of alcohol a. Alcohol + oxidizing agent COOH (1, /Aldehyde (1, partial)/Ketone (2) b. Reaction condition: aqueous, acidic solution. The carboxylic acid and the aldehyde vapor aldehyde can be obtained through different experimental set-ups i. Distill to get aldehyde 1. To distill you heat up the solution and collect the boling solution vapor complete) water out condensing tube water in ii. Heat under reflux to get COOH condensing tube 1. heat under reflux means you want to heat it for a long time w/o having it evaporate water in 2. to heat under reflux you need to attached a condensing tube to your flask so that vapors condense before they can escape boling solution c. Complete Oxidation: primary alcohol + oxidizing agent carboxylic acid OH + K2Cr2O7 acid O OH propanoic acid propan-1-ol d. Partial Oxidation: primary alcohol + oxidizing agent aldehyde OH + K2Cr2O7 acid O H 1-propanal propan-1-ol e. Secondary alcohol + oxidizing agent ketone OH butan-2-ol + acid O K2Cr2O7 butan-2-one 9. Condensation of alcohol a. Condensation of alcohol alkene b. Reaction conditions: i. 170 and concentrated sulfuric acid or ii. H3PO4 and a catalyst or Al2O3 and a catalyst condensed aldehyde out water out vapor condenses and falls back into flask O + HO OH S O O OH HO S O H O- + H H H O H H -O HO S H O OH S OH O O + H + H O O + H H + + H H H2O