* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Giladi N.Antibodies and hybridomas
Survey
Document related concepts
Duffy antigen system wikipedia , lookup
Psychoneuroimmunology wikipedia , lookup
Lymphopoiesis wikipedia , lookup
Immune system wikipedia , lookup
DNA vaccination wikipedia , lookup
Innate immune system wikipedia , lookup
Anti-nuclear antibody wikipedia , lookup
Immunocontraception wikipedia , lookup
Adaptive immune system wikipedia , lookup
Adoptive cell transfer wikipedia , lookup
Molecular mimicry wikipedia , lookup
Cancer immunotherapy wikipedia , lookup
Polyclonal B cell response wikipedia , lookup
Transcript
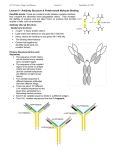
Antibodies and hybridomas Presented by Nis giladi Antibodies • Antibodies are the antigen binding protein present on the B-cell membrane and secreted by plasma cells. • Secreted antibodies circulate in the blood, where they serve as the effectors of humoral immunity by searching out and neutralizing antigens or marking them for elimination. • All antibodies share Structural features, bind to antigen, and participate in a limited number of effecter functions. Antibodies Are Heterodimers • • • • Antibody molecules have a common structure of four peptide chains. This structure consists of two identical light (L) chains, polypeptides of about 25,000 molecular weight, and two identical heavy (H) chains, larger polypeptides of molecular weight 50,000 or more. Each light chain is bound to a heavy chain by a disulfide bond, and by such noncovalent interactions as salt linkages, hydrogen bonds, and hydrophobic bonds, to form a heterodimer (H-L). Similar noncovalent interactions and disulfide bridges link the two identical heavy and light (H-L) chain combinations to each other to form the basic four-chain (H-L)2 antibody structure, a dimer of dimers. • The first 110 or so amino acids of the amino-terminal region of a light or heavy chain varies greatly among antibodies of different specificity. These segments of highly variable sequence are called V regions:VL in light chains and VH in heavy. • All of the differences in specificity displayed by different antibodies can be traced to differences in the amino acid sequences of V regions. In fact, most of the differences among antibodies fall within areas of the V regions called CDR, and it is these CDRs, on both light and heavy chains, that constitute the antigen binding site of the antibody molecule. • By contrast, within the same antibody class, far fewer differences are seen when one compares sequences throughout the rest of the molecule. The regions of relatively constant sequence beyond the variable regions have been dubbed C regions. Immunoglobulin G (IgG) • the most abundant class in serum, constitutes about 80% of the total serum immunoglobulin. • The IgG molecule consists of two heavy chains g and two k or two l light chains There are four human IgG subclasses, distinguished by differences in – chain sequence and numbered according to their decreasing average serum concentrations: IgG1, IgG2, IgG3, and IgG4. Functions: -IgG1, IgG3, and IgG4 readily cross the placenta and play an important role in protecting the developing fetus. -IgG3 is the most effective complement activator, -IgG1 and IgG3 bind with high affinity to Fc receptors on phagocytic cells and thus mediate opsonization. Immunoglobulin M (IgM) • • • • • IgM accounts for 5%–10% of the total serum immunoglobulin, Monomeric IgM, with a molecular weight of 180,000, is expressed as membrane-bound antibody on B cells. IgM is secreted by plasma cells as a pentamer in which five monomer units are held together by disulfide bonds that link their carboxyl-terminal heavy chain domains . Each pentamer contains an additional Fc-linked polypeptide called the J (joining) chain, which is disulfide-bonded to the carboxyl-terminal cysteine residue of two of the ten chains. IgM is the first immunoglobulin class produced in a primary response to an antigen, and it is also the first immunoglobulin to be synthesized by the neonate. Because of its large size, IgM does not diffuse well and therefore is found in very low concentrations in the intercellular tissue fluids. Immunoglobulin A (IgA) • Although IgA constitutes only 10%–15% of the total immunoglobulin in serum, it is the predominant immunoglobulin class in external secretions such as breast milk, saliva, tears, and mucus of the bronchial, genitourinary,and digestive tracts. In serum,IgA exists primarily as a monomer. Immunoglobulin E (IgE) • • extremely low average serum concentration (0.3 g/ml). mediate the immediate hypersensitivity reactions that are responsible for the Symptoms of hay fever, asthma, hives, and anaphylactic shock. Antibody-Mediated Effector Functions • The three major effector functions that enable antibodies to remove antigens and kill pathogens are: 1.opsonization-which promotes antigen phagocytosis by macrophages and neutrophils. 2. complement activation, which activates a pathway that leads to the generation of a collection of proteins that can perforate cell membranes. 3. Antibody dependent cell-mediated cytotoxicity (ADCC), which can kill antibody-bound target cells. In Summary •An antibody molecule consists of two identical light chains and two identical heavy chains, which are linked by disulfide bonds. •Each heavy chain has an amino-terminal variable region followed by a constant region..In any given antibody molecule, the constant region contains one of five basic heavy-chain sequences (m,d ,a ,e , or c) called isotypes and one of two basic light-chain sequences ( or ) called types. •The heavy-chain isotype determines the class of an antibody (, IgM; , IgG; , IgD; , IgA; and , IgE). • The five antibody classes have different effector functions,average serum concentrations, and half-lives. • Immunoglobulins are expressed in two forms: secreted antibody that is produced by plasma cells, and membrane-bound antibody. • Unlike polyclonal antibodies that arise from many B cell clones and have a heterogeneous collection of binding sites, a monoclonal antibody is derived from a single B cell clone and is a homogeneous collection of binding sites. Monoclonal antibodies. Monoclonal antibodies as therapy • mAbs are biomolecules that are generally indistinguishable from endogenous antibodies. • The targets of clinically useful mAbs are usually secreted molecules such as cytokines or the extracellular portions of transmembrane proteins such as growth factor receptors (GFRs) and adhesion molecules. • Such extracellular targets are representative of relevant targets in a wide variety of diseases. Examples include ErbB family members and single-pass receptor tyrosine kinases (cancer);tumor necrosis factor (TNF)-a and other cytokines (inflammatory diseases such as ulcerative colitis, rheumatoid arthritis, juvenile rheumatoid arthritis, ankylosis spondylitis,psoriatic arthritis, and psoriasis. • Because the patient’s immune response is limiting the therapeutic utility of mAbs there are major effort to reduce inherent mAb immunogenicity. by production of chimeric and Humanized mAbs . • Several chimeric mAbs (eg, rituximab, cetuximab ,Erbitux ) have been associated with markedly fewer AARs compared with murine mAbs. Nevertheless, the development of the human anti-chimeric antibody (HACA) response remains a potentially significant problem that requires close monitoring and. HACA response rates of up to 61% have been reported with infliximab and have been associated with shorter duration of effect and increased risk for infusion reactions. • Reducing the immunogenicity associated with mAbs is a critical goal. Because of immunogenicity, the successful use of many murine mAbs has been restricted to acute indications (eg, acute graft rejection, imaging applications). • The next step in mAb evolution referred to as ‘‘humanization’’ intended to replace all rodent sequences except the CDRs regions with human sequences. Human Monoclonal Technologies 1.Hybridoma 1.Mice are immunized with antigen A and given an intravenous booster immunization three days before they are killed, in order to produce a large population of spleen cells secreting specific antibody. Spleen cells die after a few days in culture. In order to produce a continuous source of antibody. 2. Spleen cells are fused with immortal myeloma cells by using polyethylene glycol (PEG) to produce a hybrid cell line called a hybridoma. 3.The myeloma cells are selected by HAT medium because they lack the enzyme hypoxanthine:guanine phosphoribosyl transferase (HGPRT). The HGPRT gene contributed by the spleen cell allows hybrid cells to survive in the HAT medium, and only hybrid cells can grow continuously in culture because of the malignant potential contributed by the myeloma cells. Therefore, unfused myeloma cells and unfused spleen cells die in the HAT medium. 4.Individual hybridomas are then screened for antibody production, and cells that make antibody of the desired specificity are cloned by growing them up from a single antibodyproducing cell. 5. The cloned hybridoma cells are grown in bulk culture to produce large amounts of antibody. As each hybridoma is descended from a single cell, all the cells of a hybridoma cell line make the same antibody molecule, which is thus called a monoclonal antibody. • 2. Xenografting transplantation in SCID • One way to circumvent murine sequence-related complications is to reconstitute a human immune system in severe combined immune deficiency (SCID) mice. • By xenografting fetal human tissues in these mice, it is possible to recapitulate the diversity of the human immune system within an animal that can be immunized and killed for retrieval of splenocytes for subsequent fusion. • The major disadvantagein that the human xenograft is not passed on through the germ line, necessitating regeneration of xenograft mice for development of each specific mAb. • Two alternative strategies now at the forefront of human mAb production largely circumvent the need for human donors and are contributing numerous therapeutic molecules and candidates. 3.Phage Display-Based Generation of Human Antibodies • The technique takes advantage of filamentous phage (bacterial viruses) to isolate genes based on their protein products Phage display synthesis of human mAbs. Variable (VH and VL) domains from hybridomas or pools of B cells are cloned by reverse transcriptase polymerase chain reaction (PCR). The PCR fragments are inserted into gene III offilamentous phage. The domains are joined by a short peptide linker and associate via framework regionmediated interactions,generating a scFv. Display of the pIII-scFv fusion on the phage coat allows it to interact with antigen. High-affinity scFvs bind phage to a immobilized antigen during multiple highstringency washes, whereas low-affinity phage are removed (‘‘panning’’). Adherent phage are then eluted and used to infect Escherichia coli. The scFv can then be altered through directed or random mutagenesis, creating subpools of phage with novel binding characteristics. When optimized, the VH and VL domains can then be cloned into heavy and light chain expression vectors and transfected into hybridoma cells for expression of conventional mAbs. 4.Fully Human Antibodies From Transgenic Mice • Generation of transgenic mice in which the murine immunoglobulin genes have been disrupted and replaced with human immunoglobulin gene clusters. • Conventional transgenic strategies had to be adapted, because the immunoglobulin genes are arranged in large clusters, rendering their cloning quite arduous:the human heavy, l-light, and k-light chain loci,located on chromosomes 14, 22, and 2, respectively, span more than 1 megabase each. D end