* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download `background radiation`.
Radiation therapy wikipedia , lookup
Gamma spectroscopy wikipedia , lookup
Fallout shelter wikipedia , lookup
Nuclear binding energy wikipedia , lookup
Valley of stability wikipedia , lookup
Technetium-99m wikipedia , lookup
Background radiation wikipedia , lookup
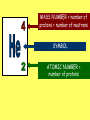
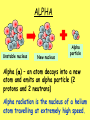
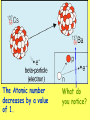
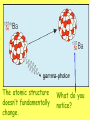
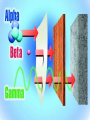
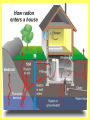
Radioactivity Learning objectives: To be able to recall and explain the structure and properties of the atom. To be able to recall the three main types of radiation. To be able to explain the structure and properties of the three main forms of radiation. Everything is made up of miniscule particles called ATOMS. Inside atoms are even smaller particles. Can you name the parts of the atom? ELECTRONS orbit the nucleus This is the NUCLEUS. PROTONS and NEUTRONS are found in the nucleus PARTICLE Proton Neutron Electron RELATIVE MASS 1 1 0 CHARGE + 0 - There are usually equal numbers of Protons and Electrons in the atom. WHY? This is because they carry opposite charges. If you have an equal number of + and – charges they will cancel each other out so we get a balanced or neutral atom. Is this a stable (balanced) atom? Why? What is the MASS NUMBER? 5 (three Protons & two Neutrons) MASS NUMBER = number of protons + number of neutrons SYMBOL ATOMIC NUMBER = number of protons RADIOACTIVE MATERIALS Most atoms are stable. However, radioactive atoms are not – they are unbalanced and unstable. They ‘want’ to become stable (balanced). So to try to achieve this state they emit (give out) energy in the form of radiation. This releases ENERGY. Sometimes a MASSIVE amount of energy. Can you think of any examples? We’ll look at examples and uses in more detail in another lesson. TYPES OF RADIATION How many types of radiation are there and how are they different? What are they? What do you notice? So, what is an alpha particle? ALPHA Unstable nucleus New nucleus Alpha particle Alpha () – an atom decays into a new atom and emits an alpha particle (2 protons and 2 neutrons) Alpha radiation is the nucleus of a helium atom travelling at extremely high speed. The Atomic number decreases by a value of 1. What do you notice? BETA Unstable nucleus New nucleus Beta particle Beta () – an atom decays into a new atom by changing a neutron into a proton and electron. The fast moving, high energy electron is called a beta particle. The atomic structure doesn’t fundamentally change. What do you notice? GAMMA Unstable nucleus New nucleus Gamma radiation Gamma – after or decay surplus energy is sometimes emitted. The atom itself is not changed. Gamma radiation is part of the EM Spectrum; a wave with a very high frequency, very short wavelength. Types of radiation Unstable nucleus New nucleus Alpha particle Beta particle Unstable nucleus New nucleus Unstable nucleus New nucleus Gamma radiation Alpha () – a high speed helium nucleus is emitted (2 protons & 2 neutrons). Beta () – the fast moving, high energy electron is called a beta particle. Gamma (g) – after or decay surplus energy is sometimes emitted. Gamma radiation is a wave with very high frequency with short wavelength. Type of radiation Symbol What is it made from? Alpha Helium nucleus. 2 protons & 2 neutrons Beta High speed electron Gamma g High energy wave How far will it travel in air? What stops it? PROPERTIES Alpha, Beta and Gamma all have different properties: • They travel different distances • Different strengths • They are stopped by different materials. DEMO… Radioactivity Copy the diagram below and complete the arrows for each type. Then use the words at the bottom to construct an explanation in your own words. g Alpha, Beta, Gamma, Thick lead, Paper, Aluminium Type of radiation Symbol What is it made from? How far will it travel in air? cm What stops it? Alpha Helium nucleus. 2 protons & 2 neutrons Beta High speed m electron Aluminium Gamma g High energy wave Thick lead & concrete Lots of m Air /paper Quiz 1.What do you call the centre of an atom? A) Electron B) Nucleus C) Proton D) Neutron Quiz 2.A stable atom has… A) Equal numbers of Protons & Electrons B) Different numbers of Protons C) Equal numbers of Electrons & Neutrons D) Equal numbers of Protons & Electrons Quiz 3.How many types of radiation are there? A) 3 B) 9 C) 2 D) 4 Quiz 4.Radiation is emitted from… A) All atoms B) Most atoms C) Unstable atoms D) Stable atoms Quiz 5.An alpha particle consists of… A) 2 protons B) A high speed electron C) A wave D) 2 protons and 2 neutrons Quiz 6.Beta radiation is… A) 2 protons B) A high speed electron C) A wave D) 2 protons and 2 neutrons Quiz 7.Gamma radiation is… A) 2 protons B) A high speed electron C) A wave D) 2 protons and 2 neutrons Quiz 8.Alpha is stopped by… A) Thick lead B) Thin paper C) A few cm of air D) Aluminium Quiz 9.Beta is stopped by… A) Aluminium B) Paper C) Lead D) Concrete Quiz 10. Gamma is mostly stopped by… A) A few cm of air B) Thick lead C) Aluminium D) Paper Learning objectives: To be able to recall and explain the structure and properties of the 3 forms of radiation. To be able to explain the ionising properties of the 3 forms of radiation. To be able to understand and explain the term ‘background radiation’ with examples. IONIZING RADIATION What does this mean? When a charged particle comes near another atom, it can pull electrons off the atom. This slows the particle down. The atom is then called an ion. If it has lost electrons, it is a positive ion. Ionisation When radiation collides with neutral atoms or molecules it alters their structure by knocking off electrons. This will leave behind IONS – this is called IONISING RADIATION. particle Electron So, what forms of radiation can be ionizing? Only a charged particle such as an or a can be ionizing. What about Gamma? Gamma has no ionizing power. But it is still the most powerful radiation but passes through most objects. BACKGROUND RADIATION Radiation is always present in the environment. We call this ‘background radiation’. There are many sources of background radiation, these include: Natural Man-made Cosmic rays, Food, Rocks (particularly granite) & Radon gas. Hospitals, Nuclear bombs and testing, Nuclear power stations & accidents 13% are man-made Background Radiation Radon gas Food Cosmic rays Gamma rays Medical Nuclear power Isotopes An isotope is an atom with a different number of neutrons: Notice that the mass number is different. How many neutrons does each isotope have? Each isotope has 8 protons – if it didn’t then it just wouldn’t be oxygen any more. A “radioisotope” is simply an isotope that is radioactive – e.g. carbon 14, which is used in carbon dating. Quick Quiz! 1.What does the term ionization mean? 2.What types of radiation cause ionization? 3.What is an isotope? 4.What is a radioisotope? Quick Quiz! 1.What does the term ionization mean? When charged particles are produced – either gaining or removing electrons from particles. 2.What types of radiation cause ionization? Only Alpha and Beta. 3.What is an isotope? Atoms of the same element with a different Mass number (i.e. different numbers of neutrons). 4.What is a radioisotope? An isotope/s of an element which emits nuclear radiation Learning objectives: To appreciate that radiation can be harmful or beneficial. To be able to explain how radiation can be used. To be able to give examples of some of the common uses. USES OF RADIATION When we think of radiation, we think DANGER! Radiation is dangerous because it damages the DNA of living cells. However, there are many ways which we can use radiation to our benefit. 1.How does radiation kill cancer cells? 2.What is the benefit of using radiation as opposed to an operation to remove a tumour? 3.Why does smoke inside the detector trigger the alarm? 4.Which type of radiation would be used to measure the thickness of a) Paper, b) Aluminium foil and C) Sheet steel? 5.Why sterilize plastic medical equipment with radiation? 6.How could a tracer be used to find a blockage in a kidney? Exam questions



















































![Atomic Structure [PowerPoint]](http://s1.studyres.com/store/data/000122096_1-1d100da6540d2f26db122fc51f672fe5-150x150.png)




