* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Affinity Chromatography
Gene expression wikipedia , lookup
Multi-state modeling of biomolecules wikipedia , lookup
Cell-penetrating peptide wikipedia , lookup
Molecular evolution wikipedia , lookup
Protein moonlighting wikipedia , lookup
Deoxyribozyme wikipedia , lookup
Community fingerprinting wikipedia , lookup
Photosynthetic reaction centre wikipedia , lookup
Two-hybrid screening wikipedia , lookup
Circular dichroism wikipedia , lookup
Nuclear magnetic resonance spectroscopy of proteins wikipedia , lookup
Signal transduction wikipedia , lookup
Intrinsically disordered proteins wikipedia , lookup
Monoclonal antibody wikipedia , lookup
Proteolysis wikipedia , lookup
List of types of proteins wikipedia , lookup
Protein–protein interaction wikipedia , lookup
Biochemistry wikipedia , lookup
Gel electrophoresis of nucleic acids wikipedia , lookup
Protein adsorption wikipedia , lookup
Protein purification wikipedia , lookup
Agarose gel electrophoresis wikipedia , lookup
Western blot wikipedia , lookup
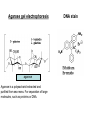
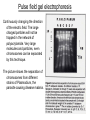
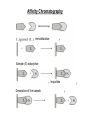
Separation techniques ? Molecules can be separated: Chemically: by charge, by action with specific reagents Physically: by solubility, by molecular weight, by density, structure (e.g. shape) Separation 1. By physical properties - Centrifugation (centrifugal force) - SDS-polyacrylamid-gellectrophoresis (charge) - Agarose-gellectrophoresis (charge) - Gelfiltration (molecular mass) 2. By chemical properties - Ion exchange chromatography - Affinity chromatography - Solubility/desalting - DNA/protein chips or microarrays 3. By physical-chemical properties Native polyacrylamid-gelelectrophoresis Paper-electrophoresis 2D-gelelectrophoresis Centrifugation 1. Differential centrifugation - Pellet/supernatant 2. Density gradient centrifugation - Continuous gradient - Step gradient Gel filtration or size exclusion chromatography Approximately half of the volume of GFC columns is occupied by the resin. Small molecules, which enter all the pores of the resin elute after one column volume. For large molecules, which can not enter any of the pores, at least half of the column volume is necessary. These molecules leave the column after half column volume. Intermediate sized molecules can enter some of the pores, but not all – they elute between the two other fractions. Ball shaped (isometric) molecules are well separated, elongated ones are difficult to separate. Why? Gel filtration does not show good separation, but can be used for desalting or for the separation of molecules of very different size. Agarose gel electrophoresis agarose Agarose is a polysacharid extracted and purified from sea moss. For separation of large molecules, such as proteins or DNA. DNA stain Agarose gel electrophoresis Pulse field gel electrophoresis Continuously changing the direction of the electric field. The large charged particles will not be trapped in the network of polyacrylamide. Very large molecules and particles, even chromosomes can be separated by this technique. The picture shows the separation of chromosomes from different strains of Plasmodium, the parasite causing disease malaria. Polyacrylamide electrophoresis (PAGE) For separaton of small molecules, such as proteins or small DNA fragments. Native-PAGE SDS-PAGE - Molecule mass - Charge - Molecule mass + SDS (sodium dodecyl sulphate) + reducing agent (e.g. mercaptoethanol) - No charge difference between molecules - No secondary structure (with reducing agent) Paper electrophoresis Electrophoresis of proteins can be carried out on wet paper. Though paper electrophoresis does not have a good resolution. However, it is a valuable tool, e.g. it can be used for the separation of native proteins. (in SDS gel electrophoresis the proteins are in denatured state). On this figure we see electrophoretograms of healthy and sick people. Isoelectric focusing separation on pH-gradient by the charge A B P h g r a d i e n t - 9 Forming a pH gradient: e.g.A With free amfolins or immobilens (weak acidic or basic molecules) 8 7 6 Amfolins - forms a pH e.g.B gradient in electronic field 5 4 + Immobilens -covalently bound to the polyacrylamide matrix 2D-electrophoresis First dimension: Isoelectric focusing Proteins in a breast cancer Second dimension: Separation by molecule mass Proteins in a healthy breast Ion exchange chromatography of proteins At slightly basic pH (8-9) most proteins are negatively charged and bind to anionic exchangers. When the ion concentration is raised, first those molecules are eluted which have the lowest number of negative side chains. Highly negatively charged proteins can be eluted only with high ionic strength solutes. The figure shows that fractions eluting at different salt concentrations contain very different proteins. Ion exchange chromatography ANIONS with exchangeable counterions starting adsorbtion buffer counterions CATIONS with exchangeable counterions start desorbtion end desorbtion separable materials regeneration gradient ions Affinity chromatography Affinity chromatography is a powerful technique to purify one component out of highly complex mixtures. It is based on the interaction of two molecules (biotin-avidin). One is fixed on the column, while the other is selected out of the mixture. A special form of affinity chromatography is metal chelate chromatography, where complexes of heavy metal ions are fixed on the column. These columns have high affinity to proteins or peptides with numerous histidine residues. Elution is carried out by imidazol compounds (His is imidazolalanine). Affinity Chromatography immobilization Sample (S) adsorption impurities Desorption of the sample Affinity separations Affinity chromatography frequently uses antibodies. Another separation technique, which is based on the use of antibodies is magnetic separation. Proteins, organelles, even intact living cells can be separated with colloidal, plastic-coated magnetic beads, carrying antibodies on their surface. The figure illustrates the principle of the separation: mRNA can also separated with Dynabeads coated with oligo(dT) primers. Why? DNA/Protein chips or microarrays For genome wide analysis. DNA chips contains single stranded DNA spots on a glass surface, capable for hybridization with the complementary strand of a cDNA clone reverse transcribed from a single mRNA. One spot represents one gene. There can be 50 000 spots or more on a glass surface. The whole genome can be screened => Genomics In protein chips there are antibodies bound to the glass surface. Each spot recognizes different protein molecules. The whole Proteome can be screened. =>Proteomics