* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download 幻灯片 1
Nucleic acid analogue wikipedia , lookup
Point mutation wikipedia , lookup
Two-hybrid screening wikipedia , lookup
Biosynthesis wikipedia , lookup
Lipid signaling wikipedia , lookup
Western blot wikipedia , lookup
Vectors in gene therapy wikipedia , lookup
Polyclonal B cell response wikipedia , lookup
Biochemical cascade wikipedia , lookup
Proteolysis wikipedia , lookup
Signal transduction wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
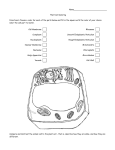
Introduction to Biochemistry (生物化学) Zhihong Li, PhD Department of Biochemistry Biochemistry • Definition: The chemistry of life – concerned with the chemical basis of life. – concerned with the various molecules that occur in living cells and organisms and with their chemical reaction. • Aim: to describe and explain, in molecular terms, all chemical processes of living cells – Structure-function – Metabolism and Regulation – How life began? Biochemistry • Significance: be essential to all life sciences as the common knowledge – – – – – Genetics; Cell biology; Molecular biology Physiology and Immunology Pharmacology and Pharmacy Toxicology; Pathology; Microbiology Zoology and Botany Cells • Basic building blocks of life. • Smallest living unit of an organism. • A cell may be an entire organism (unicellular organism) or it may be one of billions of cells that make up the organism (multicellular organism). • Grow, reproduce, use energy, adapt and respond to their environment. Cells May be Prokaryotic or Eukaryotic • Prokaryotes (Greek: pro-before; karyon-nucleus) – include various bacteria – lack a nucleus or membrane-bound structures called organelles • Eukaryotes (Greek: eu-true; karyon-nucleus) – include most other cells (plants, fungi, & animals) – have a nucleus and membranebound organelles Characteristic Bio-membranes and Organelles •Plasma Membrane-Cell’s defining boundary Providing a barrier and containing transport and signaling systems. •Nucleus – Cell’s information center Double membrane surrounding the chromosomes and the nucleolus. The place where almost all DNA replication and RNA synthesis occur. • Mitochondria- the power generators Mitochondria (Greek: mitos-thread; chondros-granule): Surrounded by a double membrane with a series of folds called cristae. Functions in energy production through metabolism. Endoplasmic reticulum (ER) – The transport network for molecules •Rough endoplasmic reticulum (RER) Covered with ribosomes (causing the "rough" appearance) which are in the process of synthesizing proteins for secretion or localization in membranes. •Ribosomes: responsible for protein synthesis •Smooth endoplasmic reticulum (SER) A site for synthesis and metabolism of lipids. •Golgi apparatus -process and package the macromolecules. A series of stacked membranes. Vesicles move between the stacks while the proteins are "processed" to a mature form. •Lysosomes-contain digestive enzyme A membrane bound organelle that is responsible for degrading proteins and membranes in the cell. •Cytoplasm enclosed by the plasma membrane, liquid portion called cytosol and it houses the membranous organelles. Biomolecules • Building blocks of cells. • Animal and plant cells contain approximately 10,000 kinds of biomolecules. – Water: constitutes 50-95% of cells contents by weight. – Ions: like Na+, K+ and Ca2+ may account for another 1%. – Organic compounds: compounds composed primarily of a Carbon skeleton. Types of biomolecules • Small molecules: – – – – Lipid, phospholipid, glycolipid, sterol Vitamin Hormone, neurotransmitter Carbohydrate, sugar • Monomers: – Amino acids – Nucleotides – Monosaccharides • Polymers: – Peptides, oligopeptides, polypeptides, proteins – Nucleic acids, i.e. DNA, RNA – Oligosaccharides, polysaccharides (including cellulose) Chemical composition of a normal man (weight 65 kg) Constituent Percent (%) Weight (kg) Water 61.6 40 Protein 17.0 11 Lipid 13.8 9 Carbohydrate 1.5 1 Minerals 6.1 4 Structural hierarchy in the molecular organization of cells Topics 1 Introduction , amino acid and protein (6h) 2 Nucleic acid (4h) 3 Carbohydrates (2h) 4 5 6 7 8 9 Vitamins (4h) Enzymes (6h) Metabolism of carbohydrates (8h) Midterm assessment Bioenergetics (4h) Metabolism of lipids (6h) 10 Metabolism of amino acids (6h) 11 Integration of metabolism and review(2h) Instructor Zhihong Li Zhihong Li Zhihong Li Zhihong Li Zhihong Li Yanlin Wang Zicheng Li Deqiao Sheng Zicheng Li Zicheng Li Text book and references • Satyanarayana U, Biochemistry. • Murray RK, Harper’s Illustrated Biochemistry, 26th ed. • Nelson DL and Cox MM. Lehninger Principles of Biochemistry, 5th ed. Some tips for study of Biochemistry • Inspiring interest, confidence • Previewing and reviewing freshly • Taking studying notes • Discussing in groups • Practice, crosstalk with other subjects • Making use of internet Final theory grade • Final theory grade= – Daily performance: 15% – Midterm assessment: 30% – Final exam: 55% • Notice: 1/3 absent, can not take part in the final exam.