* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download What is metabolism? The sum of all chemical reactions that occur as
Fatty acid metabolism wikipedia , lookup
NADH:ubiquinone oxidoreductase (H+-translocating) wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Basal metabolic rate wikipedia , lookup
Electron transport chain wikipedia , lookup
Photosynthesis wikipedia , lookup
Adenosine triphosphate wikipedia , lookup
Light-dependent reactions wikipedia , lookup
Nicotinamide adenine dinucleotide wikipedia , lookup
Photosynthetic reaction centre wikipedia , lookup
Microbial metabolism wikipedia , lookup
Citric acid cycle wikipedia , lookup
Oxidative phosphorylation wikipedia , lookup
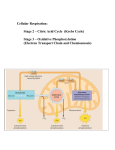
Biology 240 Metabolism What is metabolism? Biology 240 Metabolism What is energy? What is metabolism? What is energy? The sum of all chemical reactions that occur as part of the growth and development of an organism. The ability to do work make something move. What are anabolic and catabolic reactions? What is work? Anabolic = synthetic (building) Catabolic = degradative (breaking down) Making something move. Biology 240 Metabolism Energized Electrons Biology 240 Metabolism Energy in Chemical Bonds Energized Electrons 0.096 nm 25 mph O H 5 mph 11p 11n 0.109 nm C H My rendition of a car 0.154 nm C C Oxidation-Reduction Reactions C 6H 12 O6 + 6O2 Energetics and Enzymes Exergonic Reaction 6CO2 + 6H2 O Carbon is oxidized Aerobic Respiration 6CO2 + 6H2 O C3 H8 + 5O 2 Energy released Energy C 6H 12 O6 + 6O2 Oxygenic Photosynthesis Endergonic Reaction Solid Egg White Energy Energy stored Carbon is reduced Liquid Egg White 3CO 2 + 4H 2 O Fe3 + + Cu+ Fe2 + + Cu2+ Iron is reduced. Reactants Products Copper is oxidized. B B A B Products Enzymes--lower the activation energy Induced Fit Model of Enzyme Action A Reactants Types of Metabolism B A A Energy Source A Active Site A B A B Chemicals Light B Carbon Source A B A B B A Carbon Source Organic Carbon Carbon Dioxide Organic Carbon Carbon Dioxide Chemoheterotroph Chemoautotroph Photoheterotroph Photoautotroph Animals, Fungi, Some Protozoa Many bacteria Hydrogen, sulfur, iron, and nitrifying bacteria Purple and Green Nonsulfur bacteria Plants, Algae, Few Protozoa Cyanobacteria, Purple and Green Sulfur bacteria Sources of High-Energy Electrons Photosynthetic Machinery PHOTOSYSTEMS Electrons in pigment molecules energized by sunlight. Photoautotrophs Sets of pigment molecules that act as antennae for light. ELECTRON TRANSPORT SYSTEMS (ETS) Proteins, lined up in series, that transport high energy electrons like hot potatoes. Each ETS acts as an electrical wire. Electrons in pigment molecules energized by sunlight. Photoheterotrophs ATP SYNTHASE Inorganics : Electrons in large orbits (like the valence shells of metals) or in reduced molecules (like SH2, NH3 , and H2 ). Chemoautotrophs Electrons in C-C and C-H bonds of organic molecules like glucose, lipids, or proteins. Chemoheterotrophs Channel protein. Allows H+ ions to diffuse across membrane. Also acts as enzyme that synthesizes the reaction: ADP + Pi --> ATP This is an endergonic reaction. The energy needed to drive this reaction is obtained by the flow of H + ions (an electrical current). Partial view of a chloroplast (eukaryote) or plasma membrane (prokaryote) Oxygenic Photoautotrophy Anoxygenic Photoautotrophy Carbon Dioxide Fixation (Calvin-Benson Cycle) Carbon Dioxide Fixation (Calvin-Benson Cycle) H 2O H+ H+ C 6H12 O6 O2 (glucose) H H+ H+ C 6H12 O6 O2 S2 H+ H+ NADPHH + H+ H H+ ADP + Pi Partial view of a chloroplast (eukaryote) or plasma membrane (prokaryote) H+ NADP + + H+ H+ + H+ ATP 6CO 2 + 6H 2 O H+ (glucose) NADP + + H+ H+ + H 2S H 2O H+ NADPHH + H+ H+ ATP 6CO 2 + 6H 2 O ADP + Pi Partial view of a chloroplast (eukaryote) or plasma membrane (prokaryote) of Purple or Green Sulfur Bacteria Carbohydrate Catabolism: Glycolysis Photoheterotrophy C Carbon Dioxide Fixation (Calvin-Benson Cycle) Small Organics H 2O 2 + Energy released from the oxidation of the C-C bond is used to generate 4ATP. These can be used for any energetic activity the cell needs to performs. H+ H H + NADPHH + H+ H+ C C C Pyruvate C C C Pyruvate NAD + CO2 NADH NAD + ATP P A C-C bond (high-energy) is broken to form two pyruvate molecules. 2NAD + 2 One ATP forms for each H that flows through ATP Synthase . NADH The electrons taken from (oxidized) the C-C bond are added to NAD + to form NADH. C C C Pyruvate CoA C C C C C Citrate C C C C C Oxaloacetate NADH NAD + C C C C Citrate C C NAD + NAD + NADH CO2 FADH2 FAD + C C C C C Malate CO2 FAD + C C C C C Malate CO2 NAD + C C C C Succinate NADH ATP NADH CO2 FADH2 ADP In cytoplasm of procaryotes and mitochondrion of eucaryotes . NADH donates electrons (harvested from glucose) to the ETS. NAD+ H+ NADH Thus each NADH = 3ATP. C C CoA Acetyl- CoA NAD + ADP 4 + NADH CoA ATP C Chemiosmosis: Electron Transport System (Respiration) CO2 C C CoA Acetyl- CoA C C C C Succinate C C In cytoplasm of procaryotes and eucaryotes . Carbohydrate Catabolism: Krebs Cycle NADH C ADP + Pi Partial view of a chloroplast (eukaryote) or plasma membrane (prokaryote) of Purple or Green Nonsulfur Bacteria C C C Oxaloacetate C 2ATP must be burned to overcome the activation energy of the reactions. C C C Pyruvate ATP C C Fructose-1,6-diphosphate 4ADP NADP + + H+ H+ H+ C P O2 Smaller Organics (glucose) C C C Glucose 2ADP H+ 6CO 2 + 6H 2 O Small Organics ATP H+ C 6H12 O6 C NAD + H+ H+ + H FADH2 donates electrons to the second protein in the ETS. Only In AEROBIC RESPIRATION, two H + ions are pumped across. electrons that have traveled + Thus, each FADH 2 = 2ATP. through the ETS are taken up by H O 2 (these electrons are essentially waste that must be disposed of). In ANAEROBIC RESPIRATION, electrons that have traveled through the ETS are taken up by a molecule other than O 2 (these electrons are essentially waste that must be disposed of). Examples of non- O 2 electron acceptors are: S 2; SO4 ;NO 3 . H+ S 2 O2 H2 S H2 O H+ ATP ADP + Pi NADH Along plasma membrane ( procaryotes ) and inner membrane of mitochondria ( eucaryotes ). Carbohydrate Catabolism: Fermentation Energetics of Aerobic Respiration Simple or Complex Carbohydrate Aerobic Respiration C ATP Production 2 NAD C + C C C Glucose C 2ADP Glycolysis C6 H1 2O6 + 6O 2 2 NADH 38 ATP Energy Energy released (-686 kcal/mol) Energy 2 ATP C Energy stored (266 kcal/mol) C C Pyruvate 2 NAD + The process of fermentation off-loads electrons from NADH and restores the empty electron carriers, NAD + . NAD + is needed for glycolysis (which provides a net yield of 2ATP). 38ADP + 38P i 6CO 2 + 6H 2 O Reactants Products Reactants Fermentation By-Products Products Enzymes--lower the activation energy Microorganisms differ in the types of carbohydrates they can ferment. This diversity is based on the presence/absence of enzymes needed to convert these carbohydrates to glucose. Microorganisms also differ in the types of fermentation products they produce (given they can ferment a particular carbohydrate). These differences in fermentation by-products (acid or acid-gas) is also based on the presence/absence of appropriate enzymes. We make extensive use of these differences in fermentation ability when we run metabolic tests in lab. Metabolism of Other Compounds Energetics of Fermentation Triglycerides Fermentation C C C Pyruvate Glucose Proteins Pentose Phosphate Pathway ATP Production Glycerol Pyruvate Amino Acids Acetyl-CoA The 20 different amino acids are broken down and built from a variety of points within the main metabolic pathways. + 3 Fatty Acids Energy C6 H1 2O6 Energy Energy released 2C3H 4 O3 (-56 kcal/mol) 2ADP + 2Pi 2 ATP Energy stored (14 kcal/mol) Fatty acids are broken down to, and built from, acetyl- CoA . This means that fat catabolism is an aerobic process. Glycerol contributes very little of the total energy provided by fats. Nucleic Acids Krebs Cycle Reactants Products Reactants Products ETS