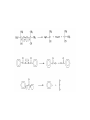* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download radicals
Ring-closing metathesis wikipedia , lookup
Marcus theory wikipedia , lookup
Woodward–Hoffmann rules wikipedia , lookup
George S. Hammond wikipedia , lookup
Physical organic chemistry wikipedia , lookup
Wolff–Kishner reduction wikipedia , lookup
Cracking (chemistry) wikipedia , lookup
Asymmetric induction wikipedia , lookup
Organosulfur compounds wikipedia , lookup
Stille reaction wikipedia , lookup
Tiffeneau–Demjanov rearrangement wikipedia , lookup
Ene reaction wikipedia , lookup
Polythiophene wikipedia , lookup
Strychnine total synthesis wikipedia , lookup
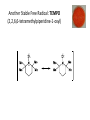
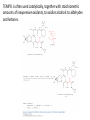
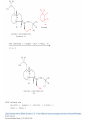
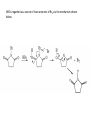
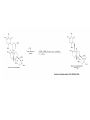
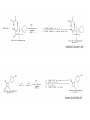
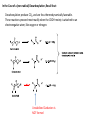
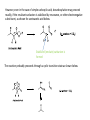
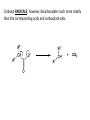
Reactions Involving Free Radicals Free radical reactions involve one electron species, frequently generated by homolysis (shown below). [Note the use of an arrow with a half head to designate the movement of one electron.] Weak bonds, like O-O bonds and X-X bonds undergo such homolysis reactions relatively easily. Hydroxy radicals (HO.) and hydrogen radicals (H.) are relatively unstable. However, radicals can be stabilized by resonance, and shielded from reaction by steric hindrance, sometimes resulting in long-lived radicals. BHT, above, is sometimes added as a food preservative, to prevent the process of auto oxidation, that occurs in the presence of air and light. Once it reacts with a peroxy radical (above) it transfers a hydrogen atom to produce a resonancestabilized, hindered oxygen radical, which does not react further. Another Stable Free Radical: TEMPO (2,2,6,6-tetramethylpiperidine-1-oxyl) TEMPO is available commercially, but expensive TEMPO is often used catalytically, together with stoichiometric amounts of inexpensive oxidants, to oxidize alcohols to aldehydes and ketones. The mechanism of this reaction involves oxidation of the N-O bond to an N=O bond by the secondary stoichiometric oxidant, which undergoes addition of the alcohol, as shown below. TEMPO is also used to initiate controlled radical polymerization reactions Geometry of Carbocation, Radical, and Carbanion Radical reactions can be divided into three steps: 1) Initiation 2) Propagation 3) Termination Weaker bonds are more readily cleaved by homolysis Some bonds can be cleaved by heating Radical Initiators AIBN Benzoyl Peroxide Link One common reaction of radicals is an atom abstraction, with a hydrogen atom being one of the most commonly abstracted atoms (note this process does NOT involve a hydrogen radical). Radical Bromination Link Allylic (and benzylic) bromination with NBS (N-Bromosuccinimide) NBS is regarded as a source of trace amounts of Br2 via the mechanism shown below. Note that the free-radical bromination occurs at the benzylic (and allylic) positions, through resonance-stabilized radical species. Reductions utilizing tributyltin hydride as a hydrogen atom donor The Sn-H bond is relatively weak (82 kcal/mole), relative to the C-H bond (99 kcal/mole) By contrast, tin forms stronger bonds to bromine, iodine, and sulfur than does carbon. C-S C-Br C-I C-H 65 kcal 68 kcal 51 kcal 99 kcal Sn-S Sn-Br Sn-I Sn-H 111 kcal 132 Kcal 56 kcal 82 kcal The use of TRIBUTYLtin derivatives has become relatively common. The three butyl groups add molecular weight to the tin and thus lower its vapor pressure. (Tin compounds can be toxic by inhalation.) While a clean, high-yielding reduction of an alkyl halide is synthetically useful, it would be even more useful if there existed a method for reducing an alcohol to an alkane. The Barton-McCombie Deoxygenation Mechanism Notice that the tin radical attacks the sulfur to generate the highly stabilized tertiary radical. (This is unlike the addition of nucleophiles to the C=O, which always occurred at the carbonyl carbon, due to the polarized nature of the carbonyl group.) Obviously the hydrogen atom is transferred from tin to carbon relatively easily. Can other alkyl groups be transferred from tin to carbon (by a free-radical process)? Radical Reaction with Allyltributylstannane One alkyl group that transfers (from tin to carbon) well is the allyl group, as shown below. Mechanism of reaction of allyltributyltin with alkyl halides (Notice that another common reaction of free radicals is addition to a C=C.) The Barton Decarboxylation In the Case of a (non-radical) Decarboxylation ,Recall that: Decarboxylation produce CO2, and are thus thermodynamically favorable. These reactions proceed most readily when the COOH moiety is attached to an electronegative atom, like oxygen or nitrogen. Unstabilized Carbanion is NOT formed However, even in the case of simple carboxylic acid, decarboxylation may proceed readily, if the resultant carbanion is stabilized by resonance, or other electronegative substituent, as shown for acetoacetic acid below. Stabilized (enolate) carbanion is formed The reaction probably proceeds through a cyclic transition state as shown below. Carboxyl RADICALS, however, decarboxylate much more readily than the corresponding acids and carboxylate salts. An early decarboxylation utilizing this fact was the Hunsdiecker reaction, shown below: Mechanism of the Hunsdiecker Reaction: Derek Barton developed a more reliable procedure for decarboxylation which does not employ the use of Br2. Mechanism of the Barton Decarboxylation: Addition of radicals to double bonds Under certain conditions, addition of H-Br (but not H-Cl or H-I) gives anti-Markovnikov regiochemistry… Why? Benzoyl Peroxide Ascaridole Morris S. Kharasch LINK Mechanistic Reason for Effect of Peroxides on the Regiochemistry of Addition of H-Br to the alkene Free Radical Polymerization Link