* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Diseases of the Immune System lec.4
Survey
Document related concepts
Protein domain wikipedia , lookup
Circular dichroism wikipedia , lookup
Protein structure prediction wikipedia , lookup
Bimolecular fluorescence complementation wikipedia , lookup
Protein folding wikipedia , lookup
Nuclear magnetic resonance spectroscopy of proteins wikipedia , lookup
Protein moonlighting wikipedia , lookup
Protein purification wikipedia , lookup
Intrinsically disordered proteins wikipedia , lookup
Protein mass spectrometry wikipedia , lookup
Western blot wikipedia , lookup
Transcript
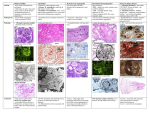
Diseases of the Immune System lec.4 2013 Amyloidosis Amyloidosis is a condition associated with a number of inherited and inflammatory disorders in which extracellular deposits of fibrillar proteins are responsible for tissue damage and functional compromise. These abnormal fibrils are produced by the aggregation of misfolded proteins or protein fragments. Pathogenesis of Amyloid Deposition All amyloid deposits are composed of nonbranching fibrils, each formed of β-sheet polypeptide chains that are wound together. The dye Congo red binds to these fibrils and produces a red–green birefringence, which is commonly used to identify amyloid deposits in tissues. Amyloidosis results from abnormal folding of proteins, which are deposited as fibrils in extracellular tissues and disrupt normal function. Normally, misfolded proteins are degraded intracellularly in proteasomes, or extracellularly by macrophages. It appears that in amyloidosis, these quality control mechanisms fail, allowing the misfolded protein to accumulate outside cells. The diverse conditions that are associated with amyloidosis all are likely to result in excessive production of proteins that are prone to misfolding. The proteins that form amyloid fall into two general categories: (1) normal proteins that have an inherent tendency to fold improperly, associate to form fibrils, and do so when they are produced in increased amounts (2) mutant proteins that are prone to misfolding and subsequent aggregation. Most common types of proteins are: 1. The AL (amyloid light chain) protein is produced by plasma cells and is made up of immunoglobulin light chains, the deposition of amyloid fibril protein of the AL type is associated with some form of monoclonal B cell proliferation. 2. The AA (amyloid-associated) fibril is a unique non-immunoglobulin protein derived from a larger serum precursor called SAA (serum amyloid-associated) protein that is synthesized in the liver, under the influence of cytokines that 1 Diseases of the Immune System lec.4 2013 are produced during inflammation; thus, long-standing inflammation leads to elevated SAA levels, and ultimately the AA form of amyloid deposits. 3. Aβ amyloid is found in the cerebral lesions of Alzheimer disease. Aβ is a peptide that constitutes the core of cerebral plaques and the amyloid deposits in cerebral blood vessels in this disease. 4. Transthyretin (TTR) is a normal serum protein that binds and transports thyroxine and retinol, hence the name. TTR is also deposited in the heart of aged persons (senile systemic amyloidosis); in such cases the protein is structurally normal, but it accumulates at high concentrations. Classification of Amyloidosis Amyloidosis is subdivided into systemic (generalized) and localized (tissuespecific) forms and is further classified on the basis of predisposing conditions. Systemic amyloidosis is associated with the following conditions: 1. Immunocyte Dyscrasias with Amyloidosis Amyloid in this category usually is systemic in distribution and is of the AL type. In some of these cases, there is a readily identifiable monoclonal plasma cell proliferation; best defined is the occurrence of systemic amyloidosis in 5% to 15% of patients with multiple myeloma. The malignant plasma cells characteristically synthesize abnormal amounts of a single specific immunoglobulin (monoclonal gammopathy), producing an M (myeloma) protein spike on serum electrophoresis. The great majority of patients with AL amyloid do not have classic multiple myeloma or any other overt B cell neoplasm; such cases are nevertheless classified as primary amyloidosis because their clinical features derive from the effects of amyloid deposition without any other associated disease. 2. Reactive Systemic Amyloidosis The amyloid deposits in this pattern are systemic in distribution and are composed of AA protein. This category was previously referred to as secondary amyloidosis, because it is secondary to an associated inflammatory condition. Classically, 2 Diseases of the Immune System lec.4 2013 tuberculosis, bronchiectasis, chronic osteomyelitis, RA, ankylosing spondylitis, inflammatory bowel disease. 3. Familial (Hereditary) Amyloidosis The best-characterized is an autosomal recessive condition called familial Mediterranean fever. This is a febrile disorder characterized by attacks of fever accompanied by inflammation of serosal surfaces, including peritoneum, pleura, and synovial membrane. It is associated with widespread tissue involvement indistinguishable from reactive systemic amyloidosis. The amyloid fibril proteins are made up of AA proteins, suggesting that this form of amyloidosis is related to the recurrent bouts of inflammation that characterize this disease. Localized Amyloidosis Sometimes deposition of amyloid is limited to a single organ or tissue without involvement of any other site in the body. The deposits may produce grossly detectable nodular masses or be evident only on microscopic examination. 1. Nodular (tumor-forming) deposits of amyloid are most often encountered in the lung, larynx, skin, urinary bladder, tongue, and the region about the eye. Frequently, there are infiltrates of lymphocytes and plasma cells in the periphery of these amyloid masses. 2. Endocrine Amyloid of localized amyloid may be found in certain endocrine tumors, such as medullary carcinoma of the thyroid gland, islet tumors of the pancreas, pheochromocytomas, and undifferentiated carcinomas of the stomach. 3. Amyloid of Aging refers to the systemic deposition of amyloid in elderly persons (usually in their 70s and 80s). Because of the dominant involvement and related dysfunction of the heart (typically manifesting as a restrictive cardiomyopathy and arrhythmias), this form also is called senile cardiac amyloidosis. The amyloid in this form is composed of normal transthyretin. 3 Diseases of the Immune System lec.4 2013 Clinical Course 1. Nonspecific complaints such as weakness, fatigue, and weight loss are the most common presenting manifestations. 2. Later in the course, amyloidosis tends to manifest in one of several ways: by renal disease, hepatomegaly, splenomegaly, or cardiac abnormalities. Renal involvement giving rise to severe proteinuria (nephrotic syndrome) often is the major cause of symptoms in reactive systemic amyloidosis. Progression of the renal disease may lead to renal failure, which is an important cause of death in amyloidosis. The hepatosplenomegaly rarely causes significant clinical dysfunction, but it may be the presenting finding. Cardiac amyloidosis may manifest as conduction disturbances or as restrictive cardiomyopathy. Cardiac arrhythmias are an important cause of death in cardiac amyloidosis. Morphology On histologic examination, the amyloid deposition is always extracellular and begins between cells, often closely adjacent to basement membranes. As the amyloid accumulates, in the AL form, perivascular and vascular localizations are common. Microscopically, routine stains reveal only amorphous, acellular, hyaline, eosinophilic extracellular material. The most commonly used staining technique uses the dye Congo red, which under ordinary light imparts a pink or red color to amyloid deposits. Under polarized light the Congo red–stained amyloid shows so called apple-green birefringence. Prognosis The outlook for patients with generalized amyloidosis is poor, with the mean survival time after diagnosis ranging from 1 to 3 years. 4