* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download IMMUNOPATHOLOGY
Lymphopoiesis wikipedia , lookup
Adaptive immune system wikipedia , lookup
Monoclonal antibody wikipedia , lookup
Psychoneuroimmunology wikipedia , lookup
Molecular mimicry wikipedia , lookup
Polyclonal B cell response wikipedia , lookup
Cancer immunotherapy wikipedia , lookup
Innate immune system wikipedia , lookup
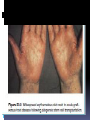
IMMUNOPATHOLOGY DR.MAYSEM LEC.4 Rejection of Tissue Transplants: -Allografts transplantation of organs from one individual to another of the same species. Rejection is a complex phenomenon involving both cell- and antibody-mediated reactions that destroy the graft. Immune Recognition of Allografts Rejection of allografts is a response mainly to MHC molecules. There are two main mechanisms by which the host immune system recognizes and responds to the MHC molecules on the graft: 1.Direct pathway: Host T cells recognize donor HLA on antigen presenting cell s (APC) derived from the donor. 2.Indirect pathway: Host T cells recognize donor HLA after processing and presention on host APC (analogous to any other exogenous processed antigen). Effector Mechanisms of Graft Rejection • Direct cytotoxic T cell (CTL-) mediated parenchymal and endothelial cytolysis • Macrophage-mediated damage • Cytokine-mediated vascular and parenchymal dysfunction • Microvascular injury also causes downstream tissue ischemia • Antibody-mediated responses can also be important; these tend to induce injury to endothelial cells rather than parenchymal cells Hyperacute rejection: Pre-formed antidonor antibodies bind to graft endothelium immediately after transplantation, leading to thrombosis, ischemic damage, and rapid graft failure Hyperacute rejection occurs when the recipient has been previously sensitized to graft antigens (e.g., by blood transfusion or pregnancy). Preformed, circulating antibody binds to graft endothelial HLA with an immediate (minutes to days) complement- and antibody -mediated injury. Acute Rejection Acute rejection typically occurs within days or months of transplantation or after cessation of immunosuppressive therapy. Both cellular and humoral mechanisms can contribute. • Acute cellular rejection T cells destroy graft parenchyma (and vessels) by cytotoxicity and inflammatory reactions. • Acute humoral rejection (rejection vasculitis) is mediated by newly synthesized (not preformed) anti-donor antibodies that cause a necrotizing vasculitis with consequent thrombosis. Chronic Rejection Chronic rejection occurs over months to years and is characterized by progressive organ dysfunction. Dominated by arteriosclerosis, this type is probably caused by T cell reaction and secretion of cytokines that induce proliferation of vascular smooth muscle cells, associated with parenchymal fibrosis Transplantation of Hematopoietic Stem Cells(HSC) Hematopoietic stem cell (HSC) transplantation is used as therapy for: 1. hematopoietic malignancies.e.g. leukemia and some non hematopoietic malignancies 2.aplastic anemias, 3. certain inherited disorders e.g. immune deficiency states and severe forms of thalassemia. Sources of Hematopoietic Stem Cells for Transplantation: 1.from donor bone marrow, 2. from the peripheral blood. 3. from the umbilical cord blood of newborns, a readily available rich source of HSCs Stem cells The recipient receives chemotherapy and/or irradiation to destroy malignant cells (e.g., in leukemia) and to create a graft bed; then, HSCs are infused into the peripheral blood, from which they home to bone marrow . Rejection of allogeneic HSC transplants seems to be mediated by some combination of host T cells and NK cells that are resistant to radiation therapy and chemotherapy. Major problems complicate this form of transplantation: 1.graft-versus-host disease(GVHD) : This occurs when immunologically competent T cells (or their precursors) are transplanted into recipients who are immunologically compromised. -Acute GVHD (occurring days to weeks after transplantation) causes epithelial cell necrosis in three principal target organs: liver, skin, and gut. -Chronic GVHD may follow the acute syndrome or may occur insidiously. The patients develop skin lesions resembling those of systemic seclerosis. 2. Immune Deficiencies. These are often of prolonged duration in recipients of HSC transplants. Recipients are susceptible to a variety of infections, mostly viral, such as cytomegalovirus (CMV) and EBV infections. THANK YOU Amyloidosis Amyloidosis is a disorder characterized by the extracellular deposits of misfolded proteins that aggregate to form insoluble fibrils. . Pathogenesis of Amyloidosis : Normally, misfolded proteins are degraded intracellularly in proteasomes, or extracellularly by macrophages. It appears that in amyloidosis, these quality control mechanisms fail, allowing the misfolded protein to accumulate outside cells. Misfolded proteins often are unstable leading to the formation of oligomers and fibrils that are deposited in tissues Proteins that form amyloid are either: • Normal proteins that have a propensity to fold improperly and associate to form fibrils; over-production (or defective catabolism) thus leads to deposition. • Mutant proteins that are prone to misfolding and aggregation; even “normal” levels of synthesis can cause deposition Of the many biochemically distinct forms of amyloid proteins that have been identified, three are most common: 1• The AL (amyloid light chain) protein is produced by plasma cells and is made up of complete immunoglobulin light chainsor the amino-terminal fragments of light chains, or both. The deposition of amyloid fibril protein of the AL type is associated with some form of monoclonal B cell proliferation 2.The AA (amyloid-associated) fibril derived from a larger serum precursor called SAA (serum amyloid-associated) protein that is synthesized in the liver under the influence of cytokines that are produced during inflammation; thus, long-standing inflammation leads to elevated SAA levels, and ultimately the AA form of amyloid deposits 3.β-Amyloid (Aβ): A peptide that forms the core of cerebral plaques and deposits within cerebral vessel walls in Alzheimer disease; it derives from a transmembrane amyloid precursor protein Other less-common forms of amyloid: • Transthyretin (TTR): A normal serum protein that binds and transports thyroxine and retinol. Excess amounts of normal TTR can deposit in hearts in senile systemic amyloidosis. while mutant forms of the protein are deposited in a group of hereditary diseases called familial amyloid polyneuropathy β2-microglobulin: component of class I HLA molecules and a normal serum protein; it is deposited in a form of amyloidosis that complicates long-term hemodialysis. Classification of Amyloidosis Amyloid may be: 1.systemic (generalized), involving several organ systems, or 2.localized, when deposits are limited to a single organ, such as the heart. On clinical grounds, the systemic, or generalized, pattern is subclassified into primary amyloidosis and secondary amyloidosis . Also there is Hereditary or familial amyloidosis Primary amyloidosis: Immunocyte dyscrasias with amyloidosis is due to AL-type amyloid; it occurs in 5% to 15% of patients with multiple myeloma Reactive secondary amyloidosis This is due to AA-type amyloid. Secondary amyloidosis is associated with chronic inflammatory states (e.g., rheumatoid arthritis, scleroderma, bronchiectasis, chronic osteomyelitis), and non-immunocyte tumors (e.g., Hodgkin lymphoma and renal cell carcinoma). Hemodialysis-associated amyloidosis: In patients on chronic hemodialysis. -due to deposition (in joints, synovium, and tendon sheaths) of β2-microglobulin not filtered by normal dialysis membranes. - Familial (Hereditary) Amyloidosis A variety of Rare familial forms of amyloidosis have been Described. The best-characterized is an autosomal recessive condition called familial Mediterranean fever characterized by attacks of fever accompanied by inflammation of serosal surfaces, including peritoneum, pleura, and synovial membrane. This disorder is encountered largely in persons of Armenian, Sephardic Jewish, and Arabic origins . - Localized Amyloidosis Sometimes deposition of amyloid is limited to a single organ or tissue without involvement of any other site in the body. . The deposits may produce grossly detectable nodular masses or be evident only on microscopic examination. Nodular (tumor-forming) deposits of amyloid are most often encountered in the lung, larynx, skin, urinary bladder, tongue, and the region about the eye Endocrine amyloid -occurs in tumors associated with hormone synthesis; for example, thyroid medullary carcinoma making procalcitonin that deposit as amyloid fibrils Amyloid of aging • occurs typically in the eighth and ninth decades and is most commonly due to deposition of nonmutant transthyretin. Although amyloid distribution is systemic, the dominant involvement is of the heart. Amyloid deposits cause tissue injury and impair normal function by causing pressure on cells and tissues. They do not evoke an inflammatory response. The diagnosis of amyloidosis 1 -Biopsy and subsequent Congo red staining is the most important tool in the diagnosis of amyloidosis. In general, biopsy is taken from the organ suspected to be involved Rectal and gingival biopsy specimens contain amyloid in as many as 75% of cases with generalized amyloidosis Haematoxylin and eosin-stained tissue : amorphous, acellular, hyaline, eosinophilic extracellular material -Congo red staining: amyloid is salmon-pink, which produces green birefringence under cross-polarized light and is the diagnostic gold standard. 2. Electrone microscope 3.In suspected cases of AL amyloidosis and immunoelectrophoresis should be performed. Bone marrow examination in such cases usually shows plasmacytosis, 4. Proteomic analysis of affected tissue is now being widely used for detection of small amounts of amyloid (from fat aspirates) and for definitive identification of the type of amyloid.