* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Diffusion Lab
Cytoplasmic streaming wikipedia , lookup
Cell nucleus wikipedia , lookup
Cell growth wikipedia , lookup
Lipid bilayer wikipedia , lookup
Cell encapsulation wikipedia , lookup
Membrane potential wikipedia , lookup
Organ-on-a-chip wikipedia , lookup
Cytokinesis wikipedia , lookup
Signal transduction wikipedia , lookup
Endomembrane system wikipedia , lookup
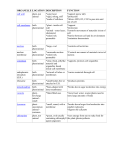
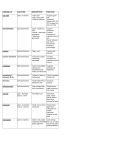
Diffusion Lab Katie Claney Period 2 11/9/09 Problem: What can get into and out of our cellophane paper? Hypothesis: If we add sugar and startch solution into a baggy, and the iodine in the beker, I think that its’ll go throght he cell membrane. Molecule Molecular Formula Starch polysaccharide made up of many glucose molecules Glucose C6H12O6 Iodine I3- Water H2O Will it cross selectively permeable membrane? no Because its so thick Yes Smaller then the starch No Why or why not? Bigger molecules No Big molecules Materials: 1.Beker 2. String 3. Cellophane paper 4. Cylender 5. Sugar slution 6. Startch solution 7. Iodine 8. test tubes Procedures: 1. Obtain one 15 cm piece of cellophane wrapping paper and two 6 inch pieces of string. 2. Soak the paper in water for a minute. 3. Make a purse out of the cellophane wrapping paper by tying a piece of string tightly to create a bag. 4. Using a pipet add 3 mL of glucose solution and 3 mL of starch solution to the bag. 5. Tie off the top of the bag with string after each bag is filled. 6. Rinse off each bag in fresh water. Be sure to rinse the string and squeeze any excess water from the string. 7. Add 50 mL of water to a 200 mL beaker. Make sure you don’t add extra water. 8. Add about 0.2 mL iodine to the water in the beaker. In the presence of starch, iodine changes color from brown to dark blue. 9. Record your observations of the solution in the bag, the solution in the beaker, and the measure you are using to evaluate movement of water in the “Initial State” row in the table below. Initial State (Before placing tube into beaker) In the tube Color Glucose? In the beaker Color Glucose? Clear, cloudy Clear Final State Yes No Measure to evaluate movement of water Empty back into the cylender Yes 10. Place the bag into the beaker. 11. After 10 minutes extract 5 ml of water from the beaker. Add 10 drops of Benedicts solution to the test water. 12. Record your observations in the “Final State” row in the table. Data/Observations: 1. Did starch move out of the bag? How do you know? No, we know because it didn dump out of the top. 2. Did iodine move into the bag? How do you know? No, it didn’t work like it was suppoessed to be 3. Did glucose move out of the bag? How do you know? No, it didn’t change colors, it couldv’e though. 4. Did water move into or out of the bag? How do you know? No, we lost water. Conclusion: 1. Using your observations fill in the chart describing which molecules passed through the membrane. Molecule Molecular Formula Did it cross selectively permeable membrane? Starch polysaccharide made up of many glucose molecules No Glucose C6H12O6 Iodine I3- Water H2O no yes yes 2. Based on the molecular structure of starch, give a possible explanation as to why starch did or did not pass through the membrane. I Think that maybe the membranes were to big, and not enough time. 3. Explain how your observations support the conclusion that cellophane wrapping paper is a selectively permeable membrane. Iodine got through it for sure, it passed through the membrane. 4. There was water on both sides of the membrane, in the tube and in the beaker. Why did water move in the direction that it did? It moved because it was forced to move. It was a small bag so high consentration, it moved to lower consentration. Each living cell is surrounded by a selectively permeable cell membrane which allows water to move into or out of the cell by diffusion. The diffusion of water across a selectively permeable membrane plays such an important role in biology that this process has been given a special name, osmosis. What will determine whether water moves into or out of the cell by osmosis? 5. Small molecules that do not have an electrical charge can easily diffuse across the selectively permeable cell membrane, but larger molecules or charged atoms or molecules (ions) cannot. Sometimes a cell needs to transport molecules that are too big or have too much charge to diffuse through the cell membrane. Special proteins embedded in the cell membrane allow certain ions and molecules to diffuse across the cell membrane. This is called facilitated diffusion. Sometimes a cell needs to move molecules from a region of lower concentration to a region of higher concentration. For example, cells need to move nutrients such as amino acids into the cell. Explain why diffusion can not be used to move amino acids from a region of lower concentration (in the fluid surrounding a cell) to a region of higher concentration (in the cytoplasm inside a cell). Special proteins embedded in the cell membrane can act as pumps to move molecules from a region of lower concentration through the cell membrane to a region of higher concentration. This type of active transport requires energy. In this investigation you used a synthetic selectively permeable membrane that is incapable of producing energy. What type of molecular transport was shown in this investigation—diffusion or active transport? Explain how you know this. *We would se facilitated diffusion, requiring energy. 6. Based on the results of this investigation, describe one important issue scientists must consider when developing a new medicine that acts on an enzyme inside cells. They need to get it across the wal. Small in size.