* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Chap9 Immunotherapy
Duffy antigen system wikipedia , lookup
Immune system wikipedia , lookup
DNA vaccination wikipedia , lookup
Monoclonal antibody wikipedia , lookup
Psychoneuroimmunology wikipedia , lookup
Adaptive immune system wikipedia , lookup
Molecular mimicry wikipedia , lookup
Innate immune system wikipedia , lookup
Immunosuppressive drug wikipedia , lookup
Polyclonal B cell response wikipedia , lookup
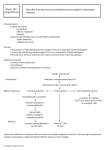
Chap 9 Immunotherapy I. Cancer Therapy Introduction Cancer therapy options: Surgery, chemotherapy and radiation therapy. Targeted therapy by Small molecule drug Ex: Imatinib (marketed by Novartis as Gleevec) is a tyrosine-kinase inhibitor used in the treatment of multiple cancers. Imatinib blocks a tyrosine kinase enzyme (BCR-Abl for phosphorylation essential in signal transduction in cancer cells) apoptosis of cancer cells. Because the BCR-Abl tyrosine kinase enzyme exists only in cancer cells and not in healthy cells, imatinib works as a form of targeted therapy—only cancer cells are killed through the drug's action. In this regard, imatinib was one of the first cancer therapies to show the potential for such targeted action, and is often cited as a paradigm for research in cancer therapeutics. Monoclonal antibody (MAb) Ex: trastuzumab (Herceptin®). Trastuzumab is a MAb that interferes with the HER2/neu receptor. Its main use is to treat certain breast cancers. The HER receptors are proteins that are embedded in the cell membrane and communicate molecular signals from outside the cell (molecules called EGFs) to inside the cell, and turn genes on and off. The HER proteins stimulate cell proliferation. In some cancers, notably certain types of breast cancer, HER2 is over-expressed, and causes cancer cells to reproduce uncontrollably. Human tumors express a number of protein antigens (tumor associated antigen, TAA) that can be recognized by the immune system, thus providing potential targets for cancer immunotherapy. However, the immune system of patients suffering from cancer is adversely affected by tumorigenesis, even early in the course of disease. Possible reasons for the resistance: most T-cells capable of recognizing self-proteins are deleted from the repertoire during T-cell differentiation in the thymus, tumor-specific self-antigens are generally weak immunogenically. 1 a natural selection process leads to the selective enrichment of clones of highly aggressive tumor cells, which no longer express cell-specific molecules. loss of MHC expression, downregulation of antigen processing machinery expression of local inhibitory molecules Mutation of epitopes at the TAA, leading to poor recognition by the immune system Cancer vaccination attempts to activate immune responses against tumor antigens. II. DC vaccination DC are professional APCs that present Ag to T cells for activation of immune responses. DC-based immunotherapy can induce antitumor immunity. However, clinical responses have been disappointing, with classic objective tumor response rates rarely exceeding 15%, but findings from emerging research indicate that DC-based vaccination might improve survival. One particular concern with immunotherapy is the possibility of induction of autoimmunity. However, cancer vaccine strategies are rarely associated with severe immune-related toxicity. First clinical trial involving DC reports that DC pulsed with a tumor antigen could elicit specific tumor-reactive T cells using the antigen expressed by non-Hodgkin’s lymphomas. In this trial, 4 patients received three DC infusion per month followed by booster injections of idiotype protein. No toxicity was observed following infusion and all patients developed strong humoral and cellular immune responses against TAA. Clinical trials of DC vaccination are under way. These studies include a broad spectrum of tumors: melanoma (黑色素瘤), colorectal (結腸), lung and renal (腎) carcinoma, prostate cancer and multiple myeloma (骨髓瘤). These studies have shown that DC vaccination may elicit tumor regression even in advanced cancer and have demonstrated the general safety of DC vaccination in cancer patients. Sources of DC Circulating immature DC precursors Representing less than 0.5% of peripheral blood mononuclear cells (PBMC). Can be isolated after 1–2 days of in vitro culture in the absence of cytokines using density gradient centrifugation. 2 The most direct and the first method utilized in clinical trial of DC therapy. Low yield of DC. In vitro differentiation of DC from CD34+ progenitors or blood monocytes Culture of adherent PBMC with the cytokines GM-CSF and IL-4 for 5–7 days leads to the generation of immature DC, which has DC markers (e.g. CD1a) and maintain the ability to take up, digest and efficiently present protein antigen to T-cells. Exposure of these immature DC to either cytokine cocktails or monocyteconditioned media results in the generation of mature DC, including ‘veiled’ morphology, high-level expression of MHC, adhesion and co-stimulatory molecules, loss of CD14 and powerful T cell stimulatory capacity. These techniques have been adapted for the generation of clinical grade DC. The antigen presenting capacity and safety (adverse effects, autoimmunity) are important parameters of DC used for immunotherapy. In all recent vaccination trials mature DC are favored over immature DC. Choice of antigen A common strategy for loading of DC with TAA explores pulsing with synthetic peptides (the immunodominant epitopes of TAA). This method requires knowledge of the amino acid sequence of relevant peptide. To avoid the generation of escape variants in solid tumors, immune response against a broad spectrum of tumor-derived epitopes is highly desirable. Mixtures of peptides directed against different epitopes. TAA in the form of purified recombinant proteins, mRNA, cDNA or virus vectors (a form of gene therapy) for antigen delivery. Pulse the DC with entire tumor lysates, total tumor RNA, whole apoptotic or necrotic tumor cells. Such DC vaccines may have better antigen-presenting properties in comparison with the peptide pulse. Two most important factors for choice of TAA: 1. elicitation of both cellular and humoral immune responsesTAA that are expressed on the surface of tumor cells are desired 3 2. Specificity for tumorwhen target antigens are not tumor-specific, but are also expressed in normal tissues severe autoimmune disease. Commercial example: Provenge (FDA approval) Consists of autologous blood APCs (mononuclear cells) pulsed with PA2024, a recombinant PAP-GM-CSF fusion protein produced using the baculovirus system. PAP (prostatic acid phosphatase) is the TAA while GM-CSF is an immune cell activator. Provenge is designed to stimulate immune responses to the prostate cancer patients. This product contains a small fraction of DC. Targets for DC immunization It is assumed that DC vaccination regimens could work effectively when the total tumor volume <100 cm3. Over this limit, DC vaccination may best be carried out as adjuvant treatment following primary surgery or chemotherapy. The primary goal of DC vaccination will be to eradicate metastatic tumor cells (which migrate from the original growth site to other locations in the body). However, the pattern of putative TAA expressed on the primary tumor may be different from that of metastasized tumor cells. The switch from local tumor growth to an invasive metastatic phenotype requires a complex series of events and numerous alterations. Route of DC delivery Many clinical studies to date have utilized iv administration. The most successful clinical trial for renal carcinoma exploited subcutaneous (皮下) application. DC vaccination trials for melanoma utilized a combination of subcutaneous and intradermal (皮內) application with iv boosting. It seems likely that iv application of DC may be effective for therapy of hematopoetic tumors and solid tumors that metastasize through blood circulation or bone marrow. In all other cases, the route of delivery depends on localization of tumor. Since mature DC do not express chemokine receptors necessary for peripheral migration, intratumoural or peritumoural routes of vaccination are of interest in cases, where a preferentially local immune response should be elicited. Thus, intradermal and subcutaneous application may be advantageous for treatment of melanoma, whereas intranodal administration may be beneficial to eliminate metastatic epithelial tumors. 4 Summary: Since DC possess all necessary components for antigen presentation, including the production of a variety of cytokines and the expression of stimulatory surface molecules, they have been termed ‘nature’s adjuvant’. Although pilot clinical trials in various cancers have yielded promising results, it appears likely that DC immunotherapy alone may have only limited success. Thus, the utilization of DC immunotherapy for management of minimal residual disease after resection of primary tumor may be promising. Two current trends: (1) developing next generation DC products with improved immunostimulatory activity; (2) Combination therapy (in combination with other therapy such as MAb) Induction of humoral immunity seems to be indispensable for treatment of many tumors. Since humoral immunity is effective only when the target structures are expressed on the surface of tumor cells, the choice of antigens for vaccination is crucial. Other options for cancer therapy Gene therapy with vectors carrying suicide genes. Gene therapy with vectors carrying anti-angiogenesis genes. Gene therapy with the replicating oncolytic virus. Adoptive cell therapy (ACT) such as adoptive transfer of T cells that are genetically engineered to produce chimeric antigen receptors (CARs) on the surface. III. CAR T cells therapy T cells contain receptors (T cell receptor, TCR) on the surface to recognize the cognate antigen. Autologous T cells are collected from the patient’s own blood. After collection, the T cells are genetically engineered to produce special receptors on their surface called CARs. CARs are proteins that allow the T cells to recognize a specific protein (antigen) on tumor cells. Domains of CARs Ectodomain 5 A signal peptide directs the nascent protein into the endoplasmic reticulum. This is essential if the receptor is to be glycosylated and anchored on the cell membrane. The antigen recognition domain: single-chain variable fragment (scFv). A spacer region links the antigen binding domain to the transmembrane domain. It should be flexible enough to allow the antigen binding domain to orient in different directions to facilitate antigen recognition. The transmembrane domain is a hydrophobic -helix that spans the membrane. Different transmembrane domains result in different receptor stability. The CD28 transmembrane domain results in a stable receptor. Endodomain: After antigen recognition, receptors cluster and a signal is transmitted to the cell. The most commonly used endodomain component is CD3zeta. This transmits an activation signal to the T cell after antigen is bound. Production and use of CAR T cells: Viral vectors are used to integrate the necessary genes into cells to deliver the genetic material needed to produce the T-cell receptors. These engineered CAR T cells are then grown and expanded. The expanded population of CAR T cells are then infused into the patient. After the infusion, the T cells can multiply in the patient’s body and, with guidance from their engineered receptor, recognize and kill cancer cells that harbor the antigen on cancer cell surface. New generation of CAR T cells: CD3-zeta may not provide a fully competent activation signal and additional co-stimulatory signaling is needed. For example, chimeric CD28 and OX40 can be used with CD3-zeta (CD3-)to transmit a proliferative/survival signal, or all three can be used together. 6 The second- and third-generation CAR typically consist of a piece of monoclonal antibody (scFv) that resides outside the T-cell membrane and is linked to stimulatory molecules (e.g. 4-1BB) inside the T cell. The scFv portion guides the cell to its target antigen. Once the T cell binds to its target antigen, the stimulatory molecules provide the necessary signals for the T cell to become fully active. In this fully active state, the T cells can more effectively proliferate and attack cancer cells. Researchers are still improving how CAR T cells are produced, including testing various delivery vectors and different stimulatory molecules to see which can help produce the most potent T cells. Two phase 1/2a trials in 2014 show that 27 out of 30 patients with relapsed or refractory cancer experience complete remissions. However, one side effect is the cytokine release syndrome (CRS, i.e. cytokine storm). CRS develops when the infused T cells proliferate as they attack a tumor, releasing a massive quantity of inflammatory cytokines (e.g. IFN-, IL-6), which can be lifethreatening. References: [1] Goldman B, DeFrancesco L. The cancer vaccine roller coaster. Nat Biotech 2009; 27:129-39. 7 IV. Appendix See Ref. [1] 8