Measuring Quantum Yields of Powder Samples
... with a Quantum Yield Measurement accessory has proven to be a useful tool for measuring quantum yields and thus aid in the effort to produce highly efficient sources of illumination. In addition to the high sensitivity and high scanning speed of the instrument, the quantum yield accessory includes a ...
... with a Quantum Yield Measurement accessory has proven to be a useful tool for measuring quantum yields and thus aid in the effort to produce highly efficient sources of illumination. In addition to the high sensitivity and high scanning speed of the instrument, the quantum yield accessory includes a ...
chapter5
... Where: red light has the longest wavelength (above 700 nm) and a relatively low energy (frequency) violet light has a shorter wavelength (around 400 nm) and a relatively high energy or frequency Atomic Spectra - When atoms absorb energy in the form of excessive heat or electrical charge, electrons m ...
... Where: red light has the longest wavelength (above 700 nm) and a relatively low energy (frequency) violet light has a shorter wavelength (around 400 nm) and a relatively high energy or frequency Atomic Spectra - When atoms absorb energy in the form of excessive heat or electrical charge, electrons m ...
Atomic Theory electron charge: -1.6 X 10-19C
... electron charge: -1.6 X 10-19 C Still, scientists did not have a clear idea of what the atom looked like. The English researcher, Ernest Rutherford, provided clearer focus when he bombarded a thin sheet of gold foil with alpha rays (beams of helium nuclei). If atoms were uniformly dense, as he expec ...
... electron charge: -1.6 X 10-19 C Still, scientists did not have a clear idea of what the atom looked like. The English researcher, Ernest Rutherford, provided clearer focus when he bombarded a thin sheet of gold foil with alpha rays (beams of helium nuclei). If atoms were uniformly dense, as he expec ...
DEPARTMENT OF PHYSICS
... Relativistic covariant formulation of electrodynamics [Jackson, Chs.11,12; Landau et al (vol. 2) (Chs.3,4)]; Radiation and energy loss from charged particles in non-uniform motions (Jackson, Ch.13,15,16); Numerical methods in solving EM problems (Jackson, Sec. 1.12, 1.13, 2.12, 5.14, ...). Topics wi ...
... Relativistic covariant formulation of electrodynamics [Jackson, Chs.11,12; Landau et al (vol. 2) (Chs.3,4)]; Radiation and energy loss from charged particles in non-uniform motions (Jackson, Ch.13,15,16); Numerical methods in solving EM problems (Jackson, Sec. 1.12, 1.13, 2.12, 5.14, ...). Topics wi ...
Chapter 38: Quantization
... Einstein framed three postulates about light quanta and their interaction with matter: 1. Light of frequency f consists of discrete quanta, each of energy E = hf, where h is Planck’s constant h = 6.63 × 10−34 J s. Each photon travels at the speed of light c = 3.00 × 108 m/s. 2. Light quanta are emit ...
... Einstein framed three postulates about light quanta and their interaction with matter: 1. Light of frequency f consists of discrete quanta, each of energy E = hf, where h is Planck’s constant h = 6.63 × 10−34 J s. Each photon travels at the speed of light c = 3.00 × 108 m/s. 2. Light quanta are emit ...
Atomic models: nuclear to quantum
... uv light is aimed at a clean metal surface, electrons are ejected from the metal, causing an electrical current. Unexpectedly, a light energy threshold was required before electrons would be emitted. In 1905, Albert Einstein applied Planck’s idea of quanta to explain the effect. • Einstein called th ...
... uv light is aimed at a clean metal surface, electrons are ejected from the metal, causing an electrical current. Unexpectedly, a light energy threshold was required before electrons would be emitted. In 1905, Albert Einstein applied Planck’s idea of quanta to explain the effect. • Einstein called th ...
File - Lenora Henderson`s Flipped Chemistry Classroom
... mathematical equation that describe the movement of an electron in the hydrogen atom The quantum mechanical model came from the mathematical solutions to the Schrodinger Equation, which is the modern description of the electrons in atoms ...
... mathematical equation that describe the movement of an electron in the hydrogen atom The quantum mechanical model came from the mathematical solutions to the Schrodinger Equation, which is the modern description of the electrons in atoms ...
class13
... dramatically, greatly decreasing resistance Superlattices are hard to mass-produce, but the effect has been seen in three-layer devices called “spin valves” The origin of giant magnetoresistance is very different from that of regular magnetoresistance! ...
... dramatically, greatly decreasing resistance Superlattices are hard to mass-produce, but the effect has been seen in three-layer devices called “spin valves” The origin of giant magnetoresistance is very different from that of regular magnetoresistance! ...
Thermal Physics Final Exam Physics 410 - 2003
... Show that this equation applies to an ideal gas (you can use results for an ideal gas without derivation; the gas does not have to be monatomic) (10 pt) 4. Consider a photon gas in a very thin cavity, so that this gas may be supposed to be two-dimensional. Assume that electromagnetic waves in the ca ...
... Show that this equation applies to an ideal gas (you can use results for an ideal gas without derivation; the gas does not have to be monatomic) (10 pt) 4. Consider a photon gas in a very thin cavity, so that this gas may be supposed to be two-dimensional. Assume that electromagnetic waves in the ca ...
File
... Electronic arrangement of Noble gases Ne 1s2 2s2 2p6 Ar 1s2 2s2 2p6 3s2 3p6 Electronic configuration of other elements can be abbreviated taking account of previous Noble gas e.g. magnesium [Ne] 3s2 ...
... Electronic arrangement of Noble gases Ne 1s2 2s2 2p6 Ar 1s2 2s2 2p6 3s2 3p6 Electronic configuration of other elements can be abbreviated taking account of previous Noble gas e.g. magnesium [Ne] 3s2 ...
Elements, mixtures and compounds lecture
... A. exists as only one type of atom: it is, therefore a pure substance (This does not often occur in nature); gold necklace? Oxygen is the most common pure element on Earth (occurs as a dioxide: O2 , what does “di” mean?) B. cannot be broken down by chemical reactions: burning/acids/eating (but nucle ...
... A. exists as only one type of atom: it is, therefore a pure substance (This does not often occur in nature); gold necklace? Oxygen is the most common pure element on Earth (occurs as a dioxide: O2 , what does “di” mean?) B. cannot be broken down by chemical reactions: burning/acids/eating (but nucle ...
supplemental_material
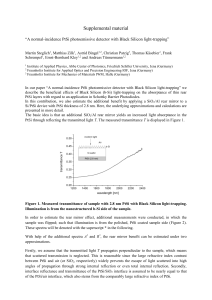
... In order to estimate the rear mirror effect, additional measurements were conducted, in which the sample was flipped; such that illumination is from the polished, PtSi coated sample side (Figure 2). These spectra will be denoted with the superscript * in the following. With help of the additional sp ...
... In order to estimate the rear mirror effect, additional measurements were conducted, in which the sample was flipped; such that illumination is from the polished, PtSi coated sample side (Figure 2). These spectra will be denoted with the superscript * in the following. With help of the additional sp ...
Topic 3&4 Atoms and the per.table
... Q4. Explain why the metal elements in group 1 are (a) called the alkali metals. (b) stored under oil. Q5. What happens to the melting point of the elements in group 7 (the halogens) as you go the group? Answers:- Q3. Lithium. Q4. (a) The elements in group 1 react with water to form an ...
... Q4. Explain why the metal elements in group 1 are (a) called the alkali metals. (b) stored under oil. Q5. What happens to the melting point of the elements in group 7 (the halogens) as you go the group? Answers:- Q3. Lithium. Q4. (a) The elements in group 1 react with water to form an ...
Chemical Terms and Keywords
... This review sheet contains an alphabetical list of chemical terms, keywords, and equations used or discussed in Chemistry 130. For each term or keyword, you should be able to write a few sentences about the topic and its relationships to other topics in the same area. For each equation, you should b ...
... This review sheet contains an alphabetical list of chemical terms, keywords, and equations used or discussed in Chemistry 130. For each term or keyword, you should be able to write a few sentences about the topic and its relationships to other topics in the same area. For each equation, you should b ...
Review Sheet for Benchmark Exam
... When you do an experiment do you want to control the independent variable, the dependent variable or both? ...
... When you do an experiment do you want to control the independent variable, the dependent variable or both? ...
presentation - WordPress.com
... an extra electron is added to an atom. For the bond formation electron gain enthalpy of an element should be high. ...
... an extra electron is added to an atom. For the bond formation electron gain enthalpy of an element should be high. ...
AlBr3 E IO Ionic FU C O Cov Molec C IO Cov Molec Sn E N/A N/A
... reactions: radioactive decays, nuclear fission and fusion. ...
... reactions: radioactive decays, nuclear fission and fusion. ...
Study On the Capacitance Between Orbitals and Atoms Modeling
... Figure 1 illustrates the proposed model for the Hydrogen atom (It looks like tunnel junctions turnstile). The capacitances between energy levels can be computed by substituting the first values of the line spectrum series, stated in Table 1 for Hydrogen atom, in equation (5). The capacitances C 12 t ...
... Figure 1 illustrates the proposed model for the Hydrogen atom (It looks like tunnel junctions turnstile). The capacitances between energy levels can be computed by substituting the first values of the line spectrum series, stated in Table 1 for Hydrogen atom, in equation (5). The capacitances C 12 t ...
mrnotes1 - University of Warwick
... much energy? The voltage difference is just E/e so we obtain (in round numbers 10 -3 V E / e 10V . In this case scientific practice is to multiply through by the symbol e to obtain 10-3 eV < E < 10 eV, where you can now just think of eV, or ‘electron volts’, as a unit of energy equal to 1.6 10 ...
... much energy? The voltage difference is just E/e so we obtain (in round numbers 10 -3 V E / e 10V . In this case scientific practice is to multiply through by the symbol e to obtain 10-3 eV < E < 10 eV, where you can now just think of eV, or ‘electron volts’, as a unit of energy equal to 1.6 10 ...
Introduction of New Products
... JCM-6000 NeoScopeTM let's you observe a specimen at much higher magnification than a light microscope with the same simple operation of a light microscope. The observation image appears automatically when a specimen is inserted, and you can go up to the maximum magnification of ҂60,000 quickly with ...
... JCM-6000 NeoScopeTM let's you observe a specimen at much higher magnification than a light microscope with the same simple operation of a light microscope. The observation image appears automatically when a specimen is inserted, and you can go up to the maximum magnification of ҂60,000 quickly with ...
1s 2 2s 2 2p 6 3s 2 3p 3d 4s 4p 4d 4f 5s 5p 5d 5f Ni = 28 e
... certain, specific energy level (n) orbits around the nucleus. Just as you cannot go up half a rung on a ladder, the electron could not go up a partial energy level. The electrons gained or lost enough energy to move a whole number amount of energy levels (n) away from or closer to the nucleus, or it ...
... certain, specific energy level (n) orbits around the nucleus. Just as you cannot go up half a rung on a ladder, the electron could not go up a partial energy level. The electrons gained or lost enough energy to move a whole number amount of energy levels (n) away from or closer to the nucleus, or it ...
Exam 2 Sol/81/F01
... expressions that relate τrad to atomic transition properties, you’ll find that only two such properties have changed on going from He to Ne+8. Tell me which two they are, how they change (i.e., get bigger, get smaller, get much bigger, don’t change very much, etc.), why they change the way you say t ...
... expressions that relate τrad to atomic transition properties, you’ll find that only two such properties have changed on going from He to Ne+8. Tell me which two they are, how they change (i.e., get bigger, get smaller, get much bigger, don’t change very much, etc.), why they change the way you say t ...
FREE Sample Here
... of the amplitudes, and if the two waves are in phase, you double the amplitude, which when squared means the intensity should be four times the intensity of one bulb. Don't these views contradict each other? Answer: Not really because interference varies from place to place. If the bulbs emitted ide ...
... of the amplitudes, and if the two waves are in phase, you double the amplitude, which when squared means the intensity should be four times the intensity of one bulb. Don't these views contradict each other? Answer: Not really because interference varies from place to place. If the bulbs emitted ide ...
X-ray fluorescence

X-ray fluorescence (XRF) is the emission of characteristic ""secondary"" (or fluorescent) X-rays from a material that has been excited by bombarding with high-energy X-rays or gamma rays. The phenomenon is widely used for elemental analysis and chemical analysis, particularly in the investigation of metals, glass, ceramics and building materials, and for research in geochemistry, forensic science and archaeology.