Advanced Lab: Rutherford Scattering
... We collected data over the period of four weeks ranging from angles of -60 to +65 degrees. Using the fact that radioactive decay follows a Poisson distribution, the standard deviation grows with the square root of the average number of counts. These standard deviations are shown on figure 2. Observe ...
... We collected data over the period of four weeks ranging from angles of -60 to +65 degrees. Using the fact that radioactive decay follows a Poisson distribution, the standard deviation grows with the square root of the average number of counts. These standard deviations are shown on figure 2. Observe ...
Electrons in Atoms
... • To indicate a sub-shell in a shell, the principal quantum number “n” and the angular momentum quantum number are written next to each other . For the second shell (L), the subshells s and p are indicated as 2s (n = 2, l = 0) and 2p (n = 2, l =1 ) . • Magnetic quantum number, ml: Each subshell is c ...
... • To indicate a sub-shell in a shell, the principal quantum number “n” and the angular momentum quantum number are written next to each other . For the second shell (L), the subshells s and p are indicated as 2s (n = 2, l = 0) and 2p (n = 2, l =1 ) . • Magnetic quantum number, ml: Each subshell is c ...
Electrons in Atoms
... Each element’s atomic emission spectrum is unique and can be used to determine if that element is part of an unknown compound Neon - light is produced by passing electricity through a tube filled w/ neon gas ...
... Each element’s atomic emission spectrum is unique and can be used to determine if that element is part of an unknown compound Neon - light is produced by passing electricity through a tube filled w/ neon gas ...
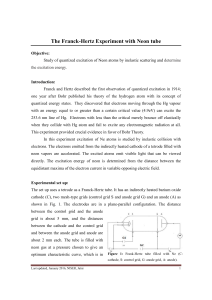
The Franck-Hertz Experiment with Neon tube
... collector current. A typical plate current versus accelerating voltage characteristics is shown in Fig. 3. As the acceleration voltage G2 is increased while UF, G1 and ...
... collector current. A typical plate current versus accelerating voltage characteristics is shown in Fig. 3. As the acceleration voltage G2 is increased while UF, G1 and ...
Wave nature of matter: de Broglie wavelength
... This is con rmed in the last two examples with the electrons. ...
... This is con rmed in the last two examples with the electrons. ...
Chapter 5 Electrons in Atoms
... Changing the energy • Heat, electricity, or light can move electron up to different energy levels. The electron is now said to be “excited” ...
... Changing the energy • Heat, electricity, or light can move electron up to different energy levels. The electron is now said to be “excited” ...
Chapter 5 Electrons in Atoms
... Changing the energy • Heat, electricity, or light can move electron up to different energy levels. The electron is now said to be “excited” ...
... Changing the energy • Heat, electricity, or light can move electron up to different energy levels. The electron is now said to be “excited” ...
Schrödinger Equation
... The Schrödinger equation plays the role of Newton's laws and conservation of energy in classical mechanics - i.e., it predicts the future behavior of a dynamic system. It is a wave equation in terms of the wavefunction which predicts analytically and precisely the probability of events or outcome. T ...
... The Schrödinger equation plays the role of Newton's laws and conservation of energy in classical mechanics - i.e., it predicts the future behavior of a dynamic system. It is a wave equation in terms of the wavefunction which predicts analytically and precisely the probability of events or outcome. T ...
Poster 1
... Upper curve: HWP set at 0° Lower curve: HWP set at 22.5° Beamsplitter effect => lower counts = half of upper counts (red curve) At 22.5°, interference is similar to the minimum of a HOM dip experiment At degeneracy, some entangled pairs interfere at PBS, so lower curve is below the red line Thus we ...
... Upper curve: HWP set at 0° Lower curve: HWP set at 22.5° Beamsplitter effect => lower counts = half of upper counts (red curve) At 22.5°, interference is similar to the minimum of a HOM dip experiment At degeneracy, some entangled pairs interfere at PBS, so lower curve is below the red line Thus we ...
Units 3 and 4 Revision
... Q4. Explain why the metal elements in group 1 are (a) called the alkali metals. (b) stored under oil. Q5. What happens to the melting point of the elements in group 7 (the halogens) as you go the group? Answers:- Q3. Lithium. Q4. (a) The elements in group 1 react with water to form an ...
... Q4. Explain why the metal elements in group 1 are (a) called the alkali metals. (b) stored under oil. Q5. What happens to the melting point of the elements in group 7 (the halogens) as you go the group? Answers:- Q3. Lithium. Q4. (a) The elements in group 1 react with water to form an ...
PowerPoint Presentation - Chapter 15
... For a neutral atom with a net charge of zero, the number of electrons outside the nucleus must equal the number of protons inside the nucleus. This is the atomic number. The total number of protons and neutrons are called mass number ...
... For a neutral atom with a net charge of zero, the number of electrons outside the nucleus must equal the number of protons inside the nucleus. This is the atomic number. The total number of protons and neutrons are called mass number ...
What is matter made of?
... System Model ~ 1913 Said that electrons orbit the nucleus along certain paths called energy levels or orbitals. Chemical properties are determined by the electrons in the outermost orbit. ...
... System Model ~ 1913 Said that electrons orbit the nucleus along certain paths called energy levels or orbitals. Chemical properties are determined by the electrons in the outermost orbit. ...
ATOMIC STRUCTURE NOTES n hcZ E ℜ
... General Periodic Trends – descending a group atomic radii increase, and with s & p blocks they decrease from left to right across period. Period 6 is different, due to lanthanide contraction. The 4f orbitals are being occupied by the lanthanides, and these have poor shielding properties. The repulsi ...
... General Periodic Trends – descending a group atomic radii increase, and with s & p blocks they decrease from left to right across period. Period 6 is different, due to lanthanide contraction. The 4f orbitals are being occupied by the lanthanides, and these have poor shielding properties. The repulsi ...
Gas Chromatography
... This is due to its high resolution, low limits of detection, speed, accuracy and reproducibility. GC can be applied to the separation of any compound that is either naturally volatile (i.e., readily goes into the gas phase) or can be converted to a volatile derivative. This makes GC useful in the se ...
... This is due to its high resolution, low limits of detection, speed, accuracy and reproducibility. GC can be applied to the separation of any compound that is either naturally volatile (i.e., readily goes into the gas phase) or can be converted to a volatile derivative. This makes GC useful in the se ...
Exam Review - hrsbstaff.ednet.ns.ca
... 25. Rutherford's observation that a gold fail scatters some alpha particle through angles greater than 90º enabled him to conclude that a) all atoms are electrically neutral. b) the nucleus of the atom contains the positive charge. c) an electron has a very small mass. d) electrons are a part of al ...
... 25. Rutherford's observation that a gold fail scatters some alpha particle through angles greater than 90º enabled him to conclude that a) all atoms are electrically neutral. b) the nucleus of the atom contains the positive charge. c) an electron has a very small mass. d) electrons are a part of al ...
File - Mr. Holz`s Website
... a. Examples of each (what foods can you find each of the macromolecules in?) b. Functions c. Basic structure (eg. Carbohydrates form carbon rings, lipids are long carbon chains, proteins fold into tangles of amino acids, nucleic acids have a sugar-phosphate backbone with A,C,G,T bases that form a tw ...
... a. Examples of each (what foods can you find each of the macromolecules in?) b. Functions c. Basic structure (eg. Carbohydrates form carbon rings, lipids are long carbon chains, proteins fold into tangles of amino acids, nucleic acids have a sugar-phosphate backbone with A,C,G,T bases that form a tw ...
Learning Goals
... for an electron in a very deep square well potential of arbitrary width in one dimension. Be able to use these distributions to predict the results of measurements of these quantities for an electron. • Explain general method for how to find allowed energies for an electron or atom in any shape pote ...
... for an electron in a very deep square well potential of arbitrary width in one dimension. Be able to use these distributions to predict the results of measurements of these quantities for an electron. • Explain general method for how to find allowed energies for an electron or atom in any shape pote ...
File
... (A) No liquid was transferred from the reaction bottle to the beaker. (B) The quantity of solid minerals decreased. (C) The cloudiness in the last bottle of limewater was caused by the product of the reaction of the colorless gas and the limewater. (D) The bubbles of gas rising from the mineral rema ...
... (A) No liquid was transferred from the reaction bottle to the beaker. (B) The quantity of solid minerals decreased. (C) The cloudiness in the last bottle of limewater was caused by the product of the reaction of the colorless gas and the limewater. (D) The bubbles of gas rising from the mineral rema ...
28 Quantum Physics
... Exercise: Electron microscope A scanning electron microscope is used to look at cell structure with 10-‐nm resoluGon. A beam of electrons from the hot filament is accelerated with a voltage of 12 kV ...
... Exercise: Electron microscope A scanning electron microscope is used to look at cell structure with 10-‐nm resoluGon. A beam of electrons from the hot filament is accelerated with a voltage of 12 kV ...
Gas Chromatography
... Molecular weights, functional groups, and polarities of component molecules are factors in selecting liquid phase. ...
... Molecular weights, functional groups, and polarities of component molecules are factors in selecting liquid phase. ...
dsst® principles of physical science i
... either used as a reference to create the exam, or were used as textbooks in college courses of the same or similar title at the time the test was developed. You may reference either the current edition of these titles or textbooks currently used at a local college or university for the same class ti ...
... either used as a reference to create the exam, or were used as textbooks in college courses of the same or similar title at the time the test was developed. You may reference either the current edition of these titles or textbooks currently used at a local college or university for the same class ti ...
X-ray fluorescence

X-ray fluorescence (XRF) is the emission of characteristic ""secondary"" (or fluorescent) X-rays from a material that has been excited by bombarding with high-energy X-rays or gamma rays. The phenomenon is widely used for elemental analysis and chemical analysis, particularly in the investigation of metals, glass, ceramics and building materials, and for research in geochemistry, forensic science and archaeology.