ECE692_3_1008
... Phonons scatter carriers, too. The higher the temperature, the worse phonon scattering. You can use the temperature dependence of conductivity or mobility to determine the contributions of various scattering mechanisms. ...
... Phonons scatter carriers, too. The higher the temperature, the worse phonon scattering. You can use the temperature dependence of conductivity or mobility to determine the contributions of various scattering mechanisms. ...
Slide 1
... •Albert Einstein had discovered that a beam of light consisted of many small packages of energy called photons. •Bohr surmised that the electrons in atoms could exist in certain discrete energy states. •Moving between these energy states would only be possible by the absorption or emission of a phot ...
... •Albert Einstein had discovered that a beam of light consisted of many small packages of energy called photons. •Bohr surmised that the electrons in atoms could exist in certain discrete energy states. •Moving between these energy states would only be possible by the absorption or emission of a phot ...
Unit 01 Qual Chem
... Physical Change = a change that does not alter the identity of a substance (shape, size, state) Chemical Change = a change in which one or more substances are converted into substances with different chemical properties ...
... Physical Change = a change that does not alter the identity of a substance (shape, size, state) Chemical Change = a change in which one or more substances are converted into substances with different chemical properties ...
J - X-ray and Observational Astronomy Group
... – Continuum produced by bremsstrahlung process as electrons are accelerated via interactions with nuclei. – Highest energy (smallest wavelength) corresponds to all the electron KE given to producing a single X-ray. – Overall shape produced by summing up of many cut-off I(λ)∝ 1/λ2 spectra. ...
... – Continuum produced by bremsstrahlung process as electrons are accelerated via interactions with nuclei. – Highest energy (smallest wavelength) corresponds to all the electron KE given to producing a single X-ray. – Overall shape produced by summing up of many cut-off I(λ)∝ 1/λ2 spectra. ...
Document
... 4. Since each orbit is of a definite fixed energy, the transition of an electron from the higher energy orbit to the lower energy orbit causes the emission of energy of a specific amount or size (a quantum). The light emitted is at a specific frequency and wavelength. ...
... 4. Since each orbit is of a definite fixed energy, the transition of an electron from the higher energy orbit to the lower energy orbit causes the emission of energy of a specific amount or size (a quantum). The light emitted is at a specific frequency and wavelength. ...
AP Chemistry Name________________________________
... TIME PROBLEMS & DIMENSIONAL ANALYSIS As chemistry students, you have two goals with problems. First, get the correct answer. Second, be able to show others WHY your answer is correct. Dimensional analysis meets both of these goals. Dimensional analysis is always a given value and one or more convers ...
... TIME PROBLEMS & DIMENSIONAL ANALYSIS As chemistry students, you have two goals with problems. First, get the correct answer. Second, be able to show others WHY your answer is correct. Dimensional analysis meets both of these goals. Dimensional analysis is always a given value and one or more convers ...
Chemistry XL-14A Nature of Light and the Atom
... (a) What is the kinetic energy of the ejected electron? (b) What is the wavelengh of the radiation that caused the photoejection of the electron? (c) What is the longest wavelength of electromagnetic radiation that could eject electrons from potassium? The work function of potassium is 2.29 eV. ...
... (a) What is the kinetic energy of the ejected electron? (b) What is the wavelengh of the radiation that caused the photoejection of the electron? (c) What is the longest wavelength of electromagnetic radiation that could eject electrons from potassium? The work function of potassium is 2.29 eV. ...
Effects of antioxidants for the degradation of flame
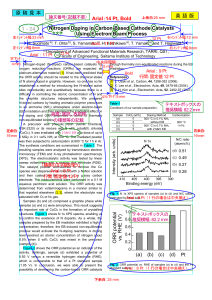
... 6 MGy in 0.1 vol% NH3 at 500 °C. The irradiated powder was then subjected to carbonization at 800 °C for 1 h in Ar. The synthesis conditions are summarized in Table 1. The resulting samples were analyzed by transmission electron microscopy (TEM) and X-ray photoelectron spectroscopy (XPS). The electr ...
... 6 MGy in 0.1 vol% NH3 at 500 °C. The irradiated powder was then subjected to carbonization at 800 °C for 1 h in Ar. The synthesis conditions are summarized in Table 1. The resulting samples were analyzed by transmission electron microscopy (TEM) and X-ray photoelectron spectroscopy (XPS). The electr ...
Isotopic indistinguishibility, scattering processes and the non mass
... will break up again into an atom and a molecule. If the isotope distribution of the leaving atom, as function of the energy transfer of the incoming atom to the complex, is different from that of the incoming atom, ozone possibly formed in presence of a stabilizing third body for a given range of en ...
... will break up again into an atom and a molecule. If the isotope distribution of the leaving atom, as function of the energy transfer of the incoming atom to the complex, is different from that of the incoming atom, ozone possibly formed in presence of a stabilizing third body for a given range of en ...
Forces between atoms and molecules
... Dispersion interaction potential: ~1/r6 Repulsion between electronic clouds at short distance: ~1/r12. ...
... Dispersion interaction potential: ~1/r6 Repulsion between electronic clouds at short distance: ~1/r12. ...
A Plausible Explanation of the double-slit Experiment in
... The 'light burst' at the detection screen (see figure) in the Tonomura double-slit experiment may not signify the arrival of "the" electron emitted from the source and going through one or the other of the two slits as a particle strikes the screen as a 'point of light'. The 'firing of an electron' ...
... The 'light burst' at the detection screen (see figure) in the Tonomura double-slit experiment may not signify the arrival of "the" electron emitted from the source and going through one or the other of the two slits as a particle strikes the screen as a 'point of light'. The 'firing of an electron' ...
PowerPoint 演示文稿
... Results of Mass Spectrometry In atomic physics,mass spectrometers are primarily of interest as instruments for analysing the isotopic composition of chemical elements. An element often has several isotopes,for example chlorine:an isotope with mass number 35 occurs with an abundance of 75.4%;the oth ...
... Results of Mass Spectrometry In atomic physics,mass spectrometers are primarily of interest as instruments for analysing the isotopic composition of chemical elements. An element often has several isotopes,for example chlorine:an isotope with mass number 35 occurs with an abundance of 75.4%;the oth ...
Quantum Mechanical Model
... Quantum Mechanical Model • As the energy of an electron increases, so does the quantum number (n) • Each principle energy level is also split up into one or more sublevels • Chart on Pg. 145 [http://www.chemistry.mcmaster.ca/esam/Chapter_4/fig4-2.jpg] ...
... Quantum Mechanical Model • As the energy of an electron increases, so does the quantum number (n) • Each principle energy level is also split up into one or more sublevels • Chart on Pg. 145 [http://www.chemistry.mcmaster.ca/esam/Chapter_4/fig4-2.jpg] ...
Can we build individual molecules atom by atom?
... ● Trapping atoms with light Lecture 2: Basic molecular physics Lecture 3: Light induced molecule formation processes ...
... ● Trapping atoms with light Lecture 2: Basic molecular physics Lecture 3: Light induced molecule formation processes ...
Chapter 10 - Lecture 3
... Structures of many-electron atoms • Because of electron correlation, no simple analytical expression for orbitals is possible • Therefore ψ(r1, r2, ….) can be expressed as ψ(r1)ψ(r2)… • Called the orbital approximation • Individual hydrogenic orbitals modified by presence of other electrons ...
... Structures of many-electron atoms • Because of electron correlation, no simple analytical expression for orbitals is possible • Therefore ψ(r1, r2, ….) can be expressed as ψ(r1)ψ(r2)… • Called the orbital approximation • Individual hydrogenic orbitals modified by presence of other electrons ...
Light forces 1. Calculation of the mean force
... 2. Give the expression of the Hamiltonian including the internal and external degrees of freedom, and the atom-field coupling in the rotating wave approximation (RWA). 3. The typical time scale for the evolution of the internal variables is set by Γ−1 . The time scale for the external variables can ...
... 2. Give the expression of the Hamiltonian including the internal and external degrees of freedom, and the atom-field coupling in the rotating wave approximation (RWA). 3. The typical time scale for the evolution of the internal variables is set by Γ−1 . The time scale for the external variables can ...
The atom:
... confused when the intensity (amplitude) of the light was increased (brighter light) and the kinetic energy of the emitted electron did not change. What happens is that as you make the light brighter more electrons are emitted but all have the same kinetic energy. The wave theory thus predicts that a ...
... confused when the intensity (amplitude) of the light was increased (brighter light) and the kinetic energy of the emitted electron did not change. What happens is that as you make the light brighter more electrons are emitted but all have the same kinetic energy. The wave theory thus predicts that a ...
Lecture Notes and Solved Problems
... cases can be examined. Such examination almost surely leads, not to the overthrow of the law, but to the discovery of other facts and laws whose action produces the apparent exceptions. As instances of such discoveries, which are in most cases due to the increasing order of accuracy made possible by ...
... cases can be examined. Such examination almost surely leads, not to the overthrow of the law, but to the discovery of other facts and laws whose action produces the apparent exceptions. As instances of such discoveries, which are in most cases due to the increasing order of accuracy made possible by ...
word-doc Practice for the final exam!
... 1. Matter in which physical state has no specific shape but does have a specific volume? a. gas b. solid c. liquid d. salts e. ice 2. A combination of sand, salt, and water is an example of a __________. a. homogeneous mixture b. heterogeneous mixture c. compound d. pure substance e. solid 3. If mat ...
... 1. Matter in which physical state has no specific shape but does have a specific volume? a. gas b. solid c. liquid d. salts e. ice 2. A combination of sand, salt, and water is an example of a __________. a. homogeneous mixture b. heterogeneous mixture c. compound d. pure substance e. solid 3. If mat ...
Structure of atoms and solids
... these free electrons to freely move throughout their crystal structure. This is not the case in covalent or ionic bonding where electrons are tightly bound to single or groups of atoms. Unlike other crystals, metals may be deformed without breaking, because the electron gas allows atoms to slide pas ...
... these free electrons to freely move throughout their crystal structure. This is not the case in covalent or ionic bonding where electrons are tightly bound to single or groups of atoms. Unlike other crystals, metals may be deformed without breaking, because the electron gas allows atoms to slide pas ...
X-ray fluorescence

X-ray fluorescence (XRF) is the emission of characteristic ""secondary"" (or fluorescent) X-rays from a material that has been excited by bombarding with high-energy X-rays or gamma rays. The phenomenon is widely used for elemental analysis and chemical analysis, particularly in the investigation of metals, glass, ceramics and building materials, and for research in geochemistry, forensic science and archaeology.