* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Forces between atoms and molecules
Aharonov–Bohm effect wikipedia , lookup
X-ray fluorescence wikipedia , lookup
X-ray photoelectron spectroscopy wikipedia , lookup
Atomic orbital wikipedia , lookup
Theoretical and experimental justification for the Schrödinger equation wikipedia , lookup
Wave–particle duality wikipedia , lookup
Rutherford backscattering spectrometry wikipedia , lookup
Molecular Hamiltonian wikipedia , lookup
Rotational–vibrational spectroscopy wikipedia , lookup
Relativistic quantum mechanics wikipedia , lookup
Tight binding wikipedia , lookup
Electron configuration wikipedia , lookup
Hydrogen atom wikipedia , lookup
Ferromagnetism wikipedia , lookup
Franck–Condon principle wikipedia , lookup
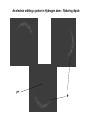
Forces between atoms and molecules Pauli exclusion principle and the Periodic Table of elements Covalent bonding http://www.estrellamountain.edu/faculty/farabee/biobk/BioBookCHEM1.html Properties of covalent compounds Ionic bonding NaCl – an ionic crystal where ions are bound by ionic bonds http://www.estrellamountain.edu/faculty/farabee/biobk/BioBookCHEM1.html Permanent dipole moment in polar molecules http://www.chem.tamu.edu/organic/Spring99/dipolemoments.html Dipole – dipole interaction Hydrogen bonding An electron orbiting a proton in Hydrogen atom – flickering dipole p+ e- Oscillating dipoles radiate – electric field The real-time evolution of the electric field of an oscillating electric dipole is shown. The dipole is located at (60,60) in the graph, oscillating at 1 rad/s (~.16Hz) in the vertical direction. http://en.wikipedia.org/wiki/Dipole#Atomic_dipoles Most of the interactions between atoms can be expressed in terms of inverse power laws of the distance, r. In most cases these analytical expressions are given in terms of potential energy of the interaction, U(r). Note that force is the negative gradient of the potential energy: F(r) = -dU(r)/dr. Coulomb potential: ~1/r Charge – dipole interaction potential: ~1/r3 Dispersion interaction potential: ~1/r6 Repulsion between electronic clouds at short distance: ~1/r12. Pauli exclusion principle No two electrons can have the same values of all four quantum numbers. Two electrons, and more generally two fermions, cannot have the same quantum state (position, momentum, mass, spin). Pauli repulsion Different terms are most important for describing the interaction between different atoms and molecules. In particular, the interaction between noble gas atoms is well described by the potential energy function, which has Lennard-Jones name: σ 12 σ 6 U(r) = 4ε [( ) − ( ) r r Here ε and σ are parameters determined from experimental data. For Argon, ε = 0.0104 eV and σ = 0.340 nm Potential energy curve for H 2 molecule Ne2 molecule