* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Effects of antioxidants for the degradation of flame
Electron configuration wikipedia , lookup
Chemical potential wikipedia , lookup
Virus quantification wikipedia , lookup
Photoredox catalysis wikipedia , lookup
Nanofluidic circuitry wikipedia , lookup
Supramolecular catalysis wikipedia , lookup
Electrolysis of water wikipedia , lookup
Artificial photosynthesis wikipedia , lookup
Diamond anvil cell wikipedia , lookup
Hydrogen-bond catalysis wikipedia , lookup
Particle-size distribution wikipedia , lookup
Inductively coupled plasma mass spectrometry wikipedia , lookup
Gas chromatography wikipedia , lookup
Vibrational analysis with scanning probe microscopy wikipedia , lookup
Allotropes of carbon wikipedia , lookup
Atomic theory wikipedia , lookup
Rutherford backscattering spectrometry wikipedia , lookup
Scanning electrochemical microscopy wikipedia , lookup
Atomic absorption spectroscopy wikipedia , lookup
Total organic carbon wikipedia , lookup
Analytical chemistry wikipedia , lookup
Radiocarbon dating wikipedia , lookup
Electrochemistry wikipedia , lookup
Community fingerprinting wikipedia , lookup
X-ray fluorescence wikipedia , lookup
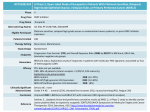
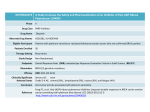
原稿見本 1 - 24 論文番号(記載不要) Arial:14 Pt, Bold 上余白:25 mm 英語版 Nitrogen Doping in Carbon-Based Cathode Catalysts 大文字 Using Electron Beam Process 右インデント幅:12 mm 12 Pt M. T. S. H. Pt T. and T. Hagiwara b) Arial:11 左インデント幅:8mm 右インデント幅:8mm a) Department of Advanced Functional Materials Research, TARRI, QST, 12 Pt Arial:11 Pt b) Faculty of Engineering, Saitama Institute of Technology 字下げ: 4 mm Nitrogen-doped (N-doped) carbon-based catalysts中央段間隔: for through thermally non-equilibrated reactions during the EB 7.5 mm oxygen reduction reactions (ORRs) are expected as irradiation. 左インデント幅:23 mm Sugimoto a), Ohta b), Yamamoto a), Koshikawa a), Yamaki a) ORR potential vs. RHE (V) Intensity (arb. units) Arial:9 Pt platinum-alternative material [1]. It has been predicted that Bold the ORR activity should be related to the chemical states References 行間:固定値 12 Pt of N atoms doped in graphite. However, no one has so far [1] J. Ozaki et al., Carbon, 44, 1298-352 (2006). established a method for introducing the N-related active [2] K. Lee et al., Electrochim. Acta, 49, 3479-85 (2004). sites reproducibly and quantitatively because there is a [3] J.-H. Kim et al., Electrochim. Acta, 52, 2492-97 (2007). difficulty in controlling the atomic concentration of N and ぶら下げ:5 mm the graphite structures independently. We prepared N-doped carbons by heating aromatic polymer precursors Table 1 テキストボックスの in an ammonia (NH3) atmosphere under electron-beam Conditions of our sample preparation. 推奨横幅:82.2 mm (EB) irradiation and then examined the irradiation effect on Sample CoCl2 (wt%) Doping Method Carbonization the doping and ORR装置・施設、放射線 properties in terms of thermally (a) 0 500 °C in 0.1% NH3 non-equilibrated kinetics.の種類等の記載 without EB (b) 5 800 °C A precursor was phenolic resin (Gunei Chemical, in N2 (c) 0 500 °C in 0.1% NH3 PSK-2320) or its mixture with 5 wt% cobalt(II) chloride with 2 MeV EB (d) 5 右余白: 左余白: (CoCl2). It was irradiated with 2 MeV EB at a dose of up to 19 mm 19 mm 6 MGy in 0.1 vol% NH3 at 500 °C. The irradiated powder N/C ratio was then subjected to carbonization at 800 °C for 1 h in Ar. N 1s (atomic%) The synthesis conditions are summarized in Table 1. The resulting samples were analyzed by transmission electron (d) 0.51 microscopy (TEM) and X-ray photoelectron spectroscopy (c) 0.49 (XPS). The electrocatalytic activity was tested by linear sweep voltammetry with a rotating disk electrode (RDE). (b) 0.27 「標準の文字数を使う」 The catalyst powder acid-washed to remove the Co species was dispersedArial:9 in a mixture (a) 0.28 Pt with a Nafion solution and then coated行間:固定値 on the surface of a glassy carbon 12 Pt 410 405 400 395 390 electrode. The measurements were performed in a 0.1 M Binding energy (eV) aqueous perchloric acid solution. The ORR activity was determined from voltammograms in a manner similar to that reported elsewhere [2, 3], where the electrolyte was Fig. 1. N 1s XPS spectra of samples (a) to (d) and N/C ratios saturated with O2 or N2 gas. estimated byArial:8 these data.Pt(1 行の場合は中央揃え) Samples (b) and (d) comprised a graphite phase while samples (a) and (c) were amorphous. This result suggests an important role of CoCl2 in the formation of crystalline 1.0 structures. Figure 1 shows N 1s XPS spectra, enabling us テキストボックスの to confirm the existence of N dopants. As a whole, the 0.8 推奨横幅:82.2 mm samples prepared by the EB irradiation exhibited a high N concentration; therefore, the EB-induced non-equilibrated 0.6 process would activate the N-doping reactions. A doping 0.4 level reached an atomic concentration of nitrogen about 0.5% when 5 wt% CoCl2 was mixed in the precursor 0.2 (sample (d)). Figure 2 shows the ORR potential as an indicator of the 0 activity. Strikingly, sample (d) exhibited a potential of (a) (b) (c) (d) Pt 0.93 V versus a reversible hydrogen electrode (RHE), which is comparable to that of a Pt standard sample Fig. 2. ORR potential vs. RHE of samples (a) to (d) and the Pt (1.05 V). In conclusion, we were able to present the standard sample. Arial:8 Pt(1 行の場合は中央揃え) possibility of developing the carbon-based ORR catalysts 下余白:25 mm Template 分類番号 1 - 24 1 カラー印刷希望 無 課題(整理)番号 使用施設・加速器 A-06 E Nitrogen Doping in Carbon-Based Cathode Catalysts Using Electron Beam Process M. Sugimoto a), T. Ohta b), S. Yamamoto a), H. Koshikawa a), T. Yamaki a) and T. Hagiwara b) a) Department of Advanced Functional Materials Research, TARRI, QST, Faculty of Engineering, Saitama Institute of Technology through thermally non-equilibrated reactions during the EB irradiation. References [1] J. Ozaki et al., Carbon, 44, 1298-352 (2006). [2] K. Lee et al., Electrochim. Acta, 49, 3479-85 (2004). [3] J.-H. Kim et al., Electrochim. Acta, 52, 2492-97 (2007). Table 1 Conditions of our sample preparation. Sample (a) (b) (c) (d) CoCl2 (wt%) 0 5 0 5 Intensity (arb. units) Nitrogen-doped (N-doped) carbon-based catalysts for oxygen reduction reactions (ORRs) are expected as platinum-alternative material [1]. It has been predicted that the ORR activity should be related to the chemical states of N atoms doped in graphite. However, no one has so far established a method for introducing the N-related active sites reproducibly and quantitatively because there is a difficulty in controlling the atomic concentration of N and the graphite structures independently. We prepared N-doped carbons by heating aromatic polymer precursors in an ammonia (NH3) atmosphere under electron-beam (EB) irradiation and then examined the irradiation effect on the doping and ORR properties in terms of thermally non-equilibrated kinetics. A precursor was phenolic resin (Gunei Chemical, PSK-2320) or its mixture with 5 wt% cobalt(II) chloride (CoCl2). It was irradiated with 2 MeV EB at a dose of up to 6 MGy in 0.1 vol% NH3 at 500 °C. The irradiated powder was then subjected to carbonization at 800 °C for 1 h in Ar. The synthesis conditions are summarized in Table 1. The resulting samples were analyzed by transmission electron microscopy (TEM) and X-ray photoelectron spectroscopy (XPS). The electrocatalytic activity was tested by linear sweep voltammetry with a rotating disk electrode (RDE). The catalyst powder acid-washed to remove the Co species was dispersed in a mixture with a Nafion solution and then coated on the surface of a glassy carbon electrode. The measurements were performed in a 0.1 M aqueous perchloric acid solution. The ORR activity was determined from voltammograms in a manner similar to that reported elsewhere [2, 3], where the electrolyte was saturated with O2 or N2 gas. Samples (b) and (d) comprised a graphite phase while samples (a) and (c) were amorphous. This result suggests an important role of CoCl2 in the formation of crystalline structures. Figure 1 shows N 1s XPS spectra, enabling us to confirm the existence of N dopants. As a whole, the samples prepared by the EB irradiation exhibited a high N concentration; therefore, the EB-induced non-equilibrated process would activate the N-doping reactions. A doping level reached an atomic concentration of nitrogen about 0.5% when 5 wt% CoCl2 was mixed in the precursor (sample (d)). Figure 2 shows the ORR potential as an indicator of the activity. Strikingly, sample (d) exhibited a potential of 0.93 V versus a reversible hydrogen electrode (RHE), which is comparable to that of a Pt standard sample (1.05 V). In conclusion, we were able to present the possibility of developing the carbon-based ORR catalysts Doping Method 500 °C in 0.1% NH3 without EB 500 °C in 0.1% NH3 with 2 MeV EB Carbonization 800 °C in N2 N/C ratio (atomic%) N 1s 410 405 400 395 (d) 0.51 (c) 0.49 (b) 0.27 (a) 0.28 390 Binding energy (eV) Fig. 1. N 1s XPS spectra of samples (a) to (d) and N/C ratios estimated by these data. ORR potential vs. RHE (V) b) 1.0 0.8 0.6 0.4 0.2 0 (a) (b) (c) (d) Pt Fig. 2. ORR potential vs. RHE of samples (a) to (d) and the Pt standard sample.