lecture CH8 A chem161pikul
... q Light as Waves, Wavelength and Frequency q The Photoelectric Effect, Light as Particles and the Relationship between Energy and Frequency q Atomic Emission and Energy Levels q The Bohr Model and its Failures q Electron Diffraction and Electrons as Waves q Quantum Numbers, Shells, Subsh ...
... q Light as Waves, Wavelength and Frequency q The Photoelectric Effect, Light as Particles and the Relationship between Energy and Frequency q Atomic Emission and Energy Levels q The Bohr Model and its Failures q Electron Diffraction and Electrons as Waves q Quantum Numbers, Shells, Subsh ...
Unit 3 - Chemistry
... • Expand without limit to fill any space. • _______________ - describes the gaseous state of a substance that is generally a liquid or solid at room temperature (different than a gas). ...
... • Expand without limit to fill any space. • _______________ - describes the gaseous state of a substance that is generally a liquid or solid at room temperature (different than a gas). ...
Einstein in 1916:" On the Quantum Theory of Radiation"
... Theory of Radiation” of 1916 [1].1 In the first part he gives a derivation of Planck’s formula which has become part of many textbooks on quantum theory. This part of the paper is now considered as the theoretical foundation of the laser, that was technically realized almost half a century later. In ...
... Theory of Radiation” of 1916 [1].1 In the first part he gives a derivation of Planck’s formula which has become part of many textbooks on quantum theory. This part of the paper is now considered as the theoretical foundation of the laser, that was technically realized almost half a century later. In ...
O.G.T. SCIENCE TEST: QUICK STUDY GUIDE Chemistry
... of atoms or the fusion (combining) of atoms. The sun produces energy by fusing hydrogen into helium. Radioactive Decay = The disintegration of an unstable atomic nucleus. Half Life = The time required for half of a sample of radioactive substance to break down by radioactive decay. Example if Carbon ...
... of atoms or the fusion (combining) of atoms. The sun produces energy by fusing hydrogen into helium. Radioactive Decay = The disintegration of an unstable atomic nucleus. Half Life = The time required for half of a sample of radioactive substance to break down by radioactive decay. Example if Carbon ...
Ionic bonding
... NaCl is a 3D array of Na+ and Cl- ions that form a repeating array, aka a crystal. • The size of the crystal depends on the number of salt units. Size can increase! How does NaCl form? Na loses an e-, and that same e- is transferred to Cl. • Both atoms become ions and are oppositely charged: Na+1 & ...
... NaCl is a 3D array of Na+ and Cl- ions that form a repeating array, aka a crystal. • The size of the crystal depends on the number of salt units. Size can increase! How does NaCl form? Na loses an e-, and that same e- is transferred to Cl. • Both atoms become ions and are oppositely charged: Na+1 & ...
The Periodic Table - Mrs Molchany`s Webpage
... across the period. More protons are added going across the period. The protons have a stronger pull on the electrons. The strong attractive forces between the protons and the outermost (valence) electrons shrinks the orbitals and makes the atoms smaller. Generally speaking, effective nuclear charge ...
... across the period. More protons are added going across the period. The protons have a stronger pull on the electrons. The strong attractive forces between the protons and the outermost (valence) electrons shrinks the orbitals and makes the atoms smaller. Generally speaking, effective nuclear charge ...
A High-Brightness Source of Narrowband, Identical
... rate expressed as a cross-correlation between detectors D1 and D2, when the write and read photons are polarization separated gwr(t) (Fig. 4A) and are allowed to interfere g45(t) (Fig. 4B). The destructive interference is most pronounced near t 0 0, and it decreases as ktk increases because the fini ...
... rate expressed as a cross-correlation between detectors D1 and D2, when the write and read photons are polarization separated gwr(t) (Fig. 4A) and are allowed to interfere g45(t) (Fig. 4B). The destructive interference is most pronounced near t 0 0, and it decreases as ktk increases because the fini ...
Dissociation energy of the C-H bond in chloroform Cl3C
... side. Replace it with the long-path cell holder. Tighten the thumbscrews. Put empty 5-mm cells in the sample and reference compartments. Use the little sliding partitions to form 5-mm cell holders. Record the background spectrum from 1650-1750 nm. Place chloroform in the sample 5-mm cell and record ...
... side. Replace it with the long-path cell holder. Tighten the thumbscrews. Put empty 5-mm cells in the sample and reference compartments. Use the little sliding partitions to form 5-mm cell holders. Record the background spectrum from 1650-1750 nm. Place chloroform in the sample 5-mm cell and record ...
Semiconductor Nanocrystals
... • Size-dependent, narrow emission spectra allows simultaneous observation of multiple processes using different colors. • The absorption spectra are continuous above band gap, so one light source excites different sized nanocrystals. • (Extra energy absorbed into thermal motion!) ...
... • Size-dependent, narrow emission spectra allows simultaneous observation of multiple processes using different colors. • The absorption spectra are continuous above band gap, so one light source excites different sized nanocrystals. • (Extra energy absorbed into thermal motion!) ...
Physics 150 Early quantum physics and photon
... Par
f0
an
electron
that
absorbs
a
whole
photon
will
be
ejected;
no
... Par
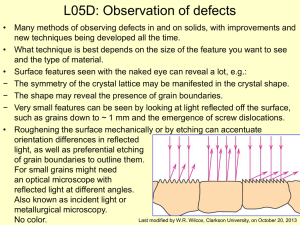
L05D - Clarkson University
... Other types of microscopy • For higher resolution need shorter wavelength than light: − X-Rays? Difficult to focus, but x-ray topography useful for dislocations − Transmitted electrons – wavelengths about 3 pm (0.003 nm) depending on energy • (Magnification up to ~1,000,000X) – Electron beam focuse ...
... Other types of microscopy • For higher resolution need shorter wavelength than light: − X-Rays? Difficult to focus, but x-ray topography useful for dislocations − Transmitted electrons – wavelengths about 3 pm (0.003 nm) depending on energy • (Magnification up to ~1,000,000X) – Electron beam focuse ...
Introduction to Feynman Diagrams and Dynamics of Interactions
... of transition amplitudes in perturbation theory. Our focus today will be on some of the concepts which unify and also which distinguish the quantum field theories of the strong, weak, and electromagnetic interactions. ...
... of transition amplitudes in perturbation theory. Our focus today will be on some of the concepts which unify and also which distinguish the quantum field theories of the strong, weak, and electromagnetic interactions. ...
Quantum/Nuclear - Issaquah Connect
... Explain how atomic spectra provide evidence for the quantization of energy in atoms ...
... Explain how atomic spectra provide evidence for the quantization of energy in atoms ...
希臘 - 中正大學化生系
... first modern chemist, and therefore one of the founders of modern chemistry, and one of the pioneers of modern experimental scientific method. 2. He endorsed the view of elements as the undecomposable constituents of material bodies; and made the distinction between mixtures and compounds. ...
... first modern chemist, and therefore one of the founders of modern chemistry, and one of the pioneers of modern experimental scientific method. 2. He endorsed the view of elements as the undecomposable constituents of material bodies; and made the distinction between mixtures and compounds. ...
IONIC BONDS MAIN GROUP CHEMISTRY
... • Reactivity of 7A nonmetals decreases as you go down group. • + metal ionic solid metal halide salt • + H2 hydrogen halide (acid in water) • + Y2 XY • + O2 nonmetal oxides. Note these oxides + water acid • + water acid ...
... • Reactivity of 7A nonmetals decreases as you go down group. • + metal ionic solid metal halide salt • + H2 hydrogen halide (acid in water) • + Y2 XY • + O2 nonmetal oxides. Note these oxides + water acid • + water acid ...
Quantum theory
... the e- will be found in a given position • The probability plots give a three dimensional shape to a region of space an eis most likely to be found ...
... the e- will be found in a given position • The probability plots give a three dimensional shape to a region of space an eis most likely to be found ...
AP Unit 1 Test Review
... (A) Atoms contain electrons. (B) Practically all the mass of an atom is contained in its nucleus. (C) Atoms contain protons, neutrons, and electrons. (D) Atoms have a positively charged nucleus surrounded by an electron cloud. (E) No two electrons in one atom can have the same four quantum numbers. ...
... (A) Atoms contain electrons. (B) Practically all the mass of an atom is contained in its nucleus. (C) Atoms contain protons, neutrons, and electrons. (D) Atoms have a positively charged nucleus surrounded by an electron cloud. (E) No two electrons in one atom can have the same four quantum numbers. ...
1 - Cobb Learning
... 4. Electrons involved in bonding between two atoms are.. A. valence electrons B. inside the nucleus C. closest to the nucleus D. positively charged 5. The modern periodic table is arranged in order of increasing … A. atomic mass B. number of valence electrons C. atomic number D. number of neutrons 6 ...
... 4. Electrons involved in bonding between two atoms are.. A. valence electrons B. inside the nucleus C. closest to the nucleus D. positively charged 5. The modern periodic table is arranged in order of increasing … A. atomic mass B. number of valence electrons C. atomic number D. number of neutrons 6 ...
الرقم الجامعي
... Q1- Which of the following electrons has the highest effective nuclear charge (Z*): a 3d and 4s electron in Mn or Co? -------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------- ...
... Q1- Which of the following electrons has the highest effective nuclear charge (Z*): a 3d and 4s electron in Mn or Co? -------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------- ...
The coherence length of black
... If quasi-monochromatic light (lc � �λ�) is applied, interference patterns consist of a great number of bright and dark quasi-monochromatic interference fringes. The order m of a bright fringe is related to the optical path difference � = m�λ� ...
... If quasi-monochromatic light (lc � �λ�) is applied, interference patterns consist of a great number of bright and dark quasi-monochromatic interference fringes. The order m of a bright fringe is related to the optical path difference � = m�λ� ...
Lab 2: Spectroscopy of Atoms and Ions
... No explanation for these lines was available, but in 1861 Robert Bunsen and Gustav Kirchhoff discovered that laboratory flames from a natural gas burner (now known as a Bunsen burner) produced characteristic bright lines from various metal salts, and that some of these lines had exactly the same wav ...
... No explanation for these lines was available, but in 1861 Robert Bunsen and Gustav Kirchhoff discovered that laboratory flames from a natural gas burner (now known as a Bunsen burner) produced characteristic bright lines from various metal salts, and that some of these lines had exactly the same wav ...
X-ray fluorescence

X-ray fluorescence (XRF) is the emission of characteristic ""secondary"" (or fluorescent) X-rays from a material that has been excited by bombarding with high-energy X-rays or gamma rays. The phenomenon is widely used for elemental analysis and chemical analysis, particularly in the investigation of metals, glass, ceramics and building materials, and for research in geochemistry, forensic science and archaeology.