Cellular respiration
... 4. Pryuvate (3 C) is converted into acetate (2 C) and releases CO2. 5. Acetate + coenzyme A yields Acetyl Coenzyme A (Acetyl-CoA). 6. Acetyl-CoA (2 C) + oxaloacetate (4 C) => citric acid (6 C). 7. Each Acetyl-CoA releases 2 CO2. A total of 6 per glucose molecule. 8. Chemical reactions of citric acid ...
... 4. Pryuvate (3 C) is converted into acetate (2 C) and releases CO2. 5. Acetate + coenzyme A yields Acetyl Coenzyme A (Acetyl-CoA). 6. Acetyl-CoA (2 C) + oxaloacetate (4 C) => citric acid (6 C). 7. Each Acetyl-CoA releases 2 CO2. A total of 6 per glucose molecule. 8. Chemical reactions of citric acid ...
Cellular Respiration
... or lactic acid. Involves 2 stages: Glycolysis Fermentation Aerobic Cellular Respiration – glucose metabolism with oxygen that produces 36 ATP molecules, CO2 and H2O. Involves 4 stages: Glycolysis Pyruvate oxidation Krebs cycle Electron transport and chemiosmosis ...
... or lactic acid. Involves 2 stages: Glycolysis Fermentation Aerobic Cellular Respiration – glucose metabolism with oxygen that produces 36 ATP molecules, CO2 and H2O. Involves 4 stages: Glycolysis Pyruvate oxidation Krebs cycle Electron transport and chemiosmosis ...
Chapter 15 The Tricarboxylic Acid Cycle
... In case of single electron carrier, semiquinones can be formed : anionic or neutral semiquinone depending on pH and on the nature of binding site when the semiquinone is bound to protein. Freely migrate on the membrane ...
... In case of single electron carrier, semiquinones can be formed : anionic or neutral semiquinone depending on pH and on the nature of binding site when the semiquinone is bound to protein. Freely migrate on the membrane ...
study sheet for chapter 9 test
... 7._____How many carbons are in each pyruvic acid molecule. 8._____How many net ATP’s are formed when one molecule of glucose breaks down in glycolysis? 9._____Number of FADH2 formed per pyruvate molecule in glycolysis? 10.____How many ATP’s are formed during the ETC and chemiosmosis? 11.____How many ...
... 7._____How many carbons are in each pyruvic acid molecule. 8._____How many net ATP’s are formed when one molecule of glucose breaks down in glycolysis? 9._____Number of FADH2 formed per pyruvate molecule in glycolysis? 10.____How many ATP’s are formed during the ETC and chemiosmosis? 11.____How many ...
Chapter 5 Quiz: Cellular respiration and fermentation Mark your
... 22) Which of the following is the organic acid produced by fermentation in human muscle cells? a. ...
... 22) Which of the following is the organic acid produced by fermentation in human muscle cells? a. ...
METABOLIC COMPARTMENTATION
... • Reduced coenzyme (NADH, 2.5 - 3 ATP or FADH2, 1.5 - 2 ATP) • The complete oxidation of glucose to carbon dioxide directly yields 2 ATP, 2 GTP, 10 NADH and 2 FADH. Depending on the assumptions used with respect to electron shuttle and ATP yield this could be the equivalent of 30 to 38 ATP molecules ...
... • Reduced coenzyme (NADH, 2.5 - 3 ATP or FADH2, 1.5 - 2 ATP) • The complete oxidation of glucose to carbon dioxide directly yields 2 ATP, 2 GTP, 10 NADH and 2 FADH. Depending on the assumptions used with respect to electron shuttle and ATP yield this could be the equivalent of 30 to 38 ATP molecules ...
Unit 3 Study Guide: Energetics
... I. UNIT VOCABULARY (This list is here to help you. You do not have to define any terms but you can if it will help you.) Chapter 8 metabolism catabolism anabolism energy (kinetic vs potential) free energy (ΔG) entropy exergonic / endergonic energy coupling ATP / ADP phosphorylation catalyst activati ...
... I. UNIT VOCABULARY (This list is here to help you. You do not have to define any terms but you can if it will help you.) Chapter 8 metabolism catabolism anabolism energy (kinetic vs potential) free energy (ΔG) entropy exergonic / endergonic energy coupling ATP / ADP phosphorylation catalyst activati ...
Growth final1 - TOP Recommended Websites
... Measuring bacterial mass (live + dead) in liquid culture ...
... Measuring bacterial mass (live + dead) in liquid culture ...
Cell Respiration
... You should now be able to: 1. Explain in general terms how redox reactions are involved in energy exchanges 2. Name the three stages of cellular respiration; for each, state the region of the eukaryotic cell where it occurs and the products that result 3. In general terms, explain the role of the e ...
... You should now be able to: 1. Explain in general terms how redox reactions are involved in energy exchanges 2. Name the three stages of cellular respiration; for each, state the region of the eukaryotic cell where it occurs and the products that result 3. In general terms, explain the role of the e ...
Cell Respiration
... You should now be able to: 1. Explain in general terms how redox reactions are involved in energy exchanges 2. Name the three stages of cellular respiration; for each, state the region of the eukaryotic cell where it occurs and the products that result 3. In general terms, explain the role of the e ...
... You should now be able to: 1. Explain in general terms how redox reactions are involved in energy exchanges 2. Name the three stages of cellular respiration; for each, state the region of the eukaryotic cell where it occurs and the products that result 3. In general terms, explain the role of the e ...
Oxidative Phosphorylation
... pyruvate + 2 ATP • High [ATP] inhibits phosphofructokinase (PFK) • High [ADP] stimulates PFK • Pasteur Effect: Increase in the rate of carbohydrate breakdown that occurs when switched from aerobic to anaerobic conditions Fig. 16-3 ...
... pyruvate + 2 ATP • High [ATP] inhibits phosphofructokinase (PFK) • High [ADP] stimulates PFK • Pasteur Effect: Increase in the rate of carbohydrate breakdown that occurs when switched from aerobic to anaerobic conditions Fig. 16-3 ...
Practice Test - IHS AP Biology
... B) the oxidation of glucose and other organic compounds. C) the H+ concentration gradient across the inner mitochondrial membrane. D) the affinity of oxygen for electrons. E) the transfer of phosphate to ADP. ...
... B) the oxidation of glucose and other organic compounds. C) the H+ concentration gradient across the inner mitochondrial membrane. D) the affinity of oxygen for electrons. E) the transfer of phosphate to ADP. ...
Electron-Transport Chain and ATP production
... Electron-Transport Chain and ATP production Occurs in the inner mitochondrial membrane where NADH and FADH2 are oxidized back to NAD+ and FAD. They transfer their e- in a series of steps and ultimately to O2: O2 + 4e- + 4H+ → 2H2O The energy released in these e- transfers is used to pump H+ (protons ...
... Electron-Transport Chain and ATP production Occurs in the inner mitochondrial membrane where NADH and FADH2 are oxidized back to NAD+ and FAD. They transfer their e- in a series of steps and ultimately to O2: O2 + 4e- + 4H+ → 2H2O The energy released in these e- transfers is used to pump H+ (protons ...
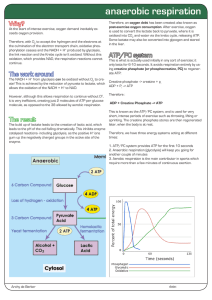
anaerobic respiration
... At the start of intense exercise, oxygen demand inevitably exceeds oxygen provision. Therefore, with O2 to accept the hydrogen and the electrons at the culmination of the electron transport chain, oxidative phosphorylation ceases and the NADH + H+ produced by glycolysis, the link reaction and the Kr ...
... At the start of intense exercise, oxygen demand inevitably exceeds oxygen provision. Therefore, with O2 to accept the hydrogen and the electrons at the culmination of the electron transport chain, oxidative phosphorylation ceases and the NADH + H+ produced by glycolysis, the link reaction and the Kr ...
Cellular Energetics
... converted to lactate (lactic acid). In this process NADH gives up its e- to form NAD+, which can now be used again for ...
... converted to lactate (lactic acid). In this process NADH gives up its e- to form NAD+, which can now be used again for ...
Cellular Energy Unit Vocabulary California Standard
... occur in specialized areas of the organism’s cells. As a basis for understanding this concept: f. f. Students know usable energy is captured from sunlight by chloroplasts and is stored through the synthesis of sugar from carbon dioxide. g. Students know the role of the mitochondria in making stored ...
... occur in specialized areas of the organism’s cells. As a basis for understanding this concept: f. f. Students know usable energy is captured from sunlight by chloroplasts and is stored through the synthesis of sugar from carbon dioxide. g. Students know the role of the mitochondria in making stored ...
File
... Acyl groups linked to ACP through phosphopantetheine prosthetic group 4. Electron carriers consume NADPH ...
... Acyl groups linked to ACP through phosphopantetheine prosthetic group 4. Electron carriers consume NADPH ...
cell respiration notes ap - Wesleyan
... Proton gradient powers ATP SYNTHASE to ADP + Pi → ATP PROTON MOTIVE FORCE = potential energy of hydrogen ion gradient CHEMIOSMOSIS = Generation of ATP from a proton gradient (It occurs in all living things) OXIDATIVE PHOSPHORYLATION using proton gradient created by electron transport chain in crista ...
... Proton gradient powers ATP SYNTHASE to ADP + Pi → ATP PROTON MOTIVE FORCE = potential energy of hydrogen ion gradient CHEMIOSMOSIS = Generation of ATP from a proton gradient (It occurs in all living things) OXIDATIVE PHOSPHORYLATION using proton gradient created by electron transport chain in crista ...
CHAPTER OUTLINE
... Inputs = 6C glucose, 2 NAD+, 2 ATP, 4 ADP +4P Outputs = 2 (3C) pyruvate, 2 NADH, 2 ADP, 4 ATP total Two ATP net gain. 7.3 Outside the Mitochondria: Fermentation Fermentation is an anaerobic pathway a cell may utilize if oxygen is limited when breaking down glucose. Advantages and Disadvantages of Fe ...
... Inputs = 6C glucose, 2 NAD+, 2 ATP, 4 ADP +4P Outputs = 2 (3C) pyruvate, 2 NADH, 2 ADP, 4 ATP total Two ATP net gain. 7.3 Outside the Mitochondria: Fermentation Fermentation is an anaerobic pathway a cell may utilize if oxygen is limited when breaking down glucose. Advantages and Disadvantages of Fe ...
METABOLISM OF CARBOHYDRATES
... 3 – phosphate ISOMERIZATON to produce more glyceraldehyde phosphate as it is used up (triose phosphate isomerase) Step #5 glyceraldehyde – 3 – phosphate + Pi + NAD+ 1,3 diphosphoglycerate + NADH OXIDATION – REDUCTION – PHOSPHORYLATION (glyceraldehyde-3-phosphate ...
... 3 – phosphate ISOMERIZATON to produce more glyceraldehyde phosphate as it is used up (triose phosphate isomerase) Step #5 glyceraldehyde – 3 – phosphate + Pi + NAD+ 1,3 diphosphoglycerate + NADH OXIDATION – REDUCTION – PHOSPHORYLATION (glyceraldehyde-3-phosphate ...
Cellular Respiration
... Splits apart a single glucose molecule (6 carbon) into two molecules of pyruvate (3 carbon) under anaerobic conditions, pyruvate converted by fermentation to lactic acid or ethanol occurs in cytoplasm pyruvate may enter mitochondria if oxygen available – breaks pyruvate down completely to CO2 and wa ...
... Splits apart a single glucose molecule (6 carbon) into two molecules of pyruvate (3 carbon) under anaerobic conditions, pyruvate converted by fermentation to lactic acid or ethanol occurs in cytoplasm pyruvate may enter mitochondria if oxygen available – breaks pyruvate down completely to CO2 and wa ...
Citric acid cycle
The citric acid cycle – also known as the tricarboxylic acid (TCA) cycle or the Krebs cycle – is a series of chemical reactions used by all aerobic organisms to generate energy through the oxidation of acetate derived from carbohydrates, fats and proteins into carbon dioxide and chemical energy in the form of adenosine triphosphate (ATP). In addition, the cycle provides precursors of certain amino acids as well as the reducing agent NADH that is used in numerous other biochemical reactions. Its central importance to many biochemical pathways suggests that it was one of the earliest established components of cellular metabolism and may have originated abiogenically.The name of this metabolic pathway is derived from citric acid (a type of tricarboxylic acid) that is consumed and then regenerated by this sequence of reactions to complete the cycle. In addition, the cycle consumes acetate (in the form of acetyl-CoA) and water, reduces NAD+ to NADH, and produces carbon dioxide as a waste byproduct. The NADH generated by the TCA cycle is fed into the oxidative phosphorylation (electron transport) pathway. The net result of these two closely linked pathways is the oxidation of nutrients to produce usable chemical energy in the form of ATP.In eukaryotic cells, the citric acid cycle occurs in the matrix of the mitochondrion. In prokaryotic cells, such as bacteria which lack mitochondria, the TCA reaction sequence is performed in the cytosol with the proton gradient for ATP production being across the cell's surface (plasma membrane) rather than the inner membrane of the mitochondrion.