CHAPTER 9
... Pre-test to identify student misconceptions prior to addressing the material covered in Chapter 9 ...
... Pre-test to identify student misconceptions prior to addressing the material covered in Chapter 9 ...
Download PDF
... The goal of this course is to learn about general aspects of biochemical pathways from the perspective of the chemical principles and chemical reactions. We will cover: 1. Biochemical structures. We will study detailed aspects of the three-dimensional structure of proteins, and how this translates i ...
... The goal of this course is to learn about general aspects of biochemical pathways from the perspective of the chemical principles and chemical reactions. We will cover: 1. Biochemical structures. We will study detailed aspects of the three-dimensional structure of proteins, and how this translates i ...
Student Version
... In a general sense, fermentation is the conversion of a carbohydrate such as sugar into an acid or an alcohol. More specifically, fermentation can refer to the use of yeast to change sugar into alcohol or the use of bacteria to create lactic acid in certain foods. Fermentation occurs naturally in ma ...
... In a general sense, fermentation is the conversion of a carbohydrate such as sugar into an acid or an alcohol. More specifically, fermentation can refer to the use of yeast to change sugar into alcohol or the use of bacteria to create lactic acid in certain foods. Fermentation occurs naturally in ma ...
Principles of BIOCHEMISTRY
... • Muscles lack pyruvate dehydrogenase and cannot produce ethanol from pyruvate • Muscle lactate dehydrogenase converts pyruvate to lactate • This reaction regenerates NAD+ for use by glyceraldehyde 3phosphate dehydrogenase in glycolysis • Lactate formed in skeletal muscles during exercise is transpo ...
... • Muscles lack pyruvate dehydrogenase and cannot produce ethanol from pyruvate • Muscle lactate dehydrogenase converts pyruvate to lactate • This reaction regenerates NAD+ for use by glyceraldehyde 3phosphate dehydrogenase in glycolysis • Lactate formed in skeletal muscles during exercise is transpo ...
QUIZ #4 LIPID STRUCTURES AND METABOLISM
... You have two 6-carbon compounds; one is glucose and the other is caproic acid (6:0). If both are complexely oxidized to CO2 and H2O, what is the ratio of their potential maximum ATPs generated? a. Glucose yields 38 ATP where as caproic acid yields 28 ATP b. Glucose yields 28 ATP where as caproic aci ...
... You have two 6-carbon compounds; one is glucose and the other is caproic acid (6:0). If both are complexely oxidized to CO2 and H2O, what is the ratio of their potential maximum ATPs generated? a. Glucose yields 38 ATP where as caproic acid yields 28 ATP b. Glucose yields 28 ATP where as caproic aci ...
Lh6Ch14aGlycolPPP
... Forwards!!!! Where is this going on in a cell? EOC Problems 1+2 can be worked from this Figure and ...
... Forwards!!!! Where is this going on in a cell? EOC Problems 1+2 can be worked from this Figure and ...
Plant Respiration Exchange of Gases in Plants - E
... Answer: Aerobic respiration takes place within the mitochondria. Following are the main steps in aerobic respiration: Stepwise removal of all the hydrogen atoms leads to complete oxidation of pyruvate. This leaves three molecules of CO2. This step takes place in the matrix of mitochondria. Electrons ...
... Answer: Aerobic respiration takes place within the mitochondria. Following are the main steps in aerobic respiration: Stepwise removal of all the hydrogen atoms leads to complete oxidation of pyruvate. This leaves three molecules of CO2. This step takes place in the matrix of mitochondria. Electrons ...
Problem Set 5 (Due February 25th) 1. Show how glucose can be
... 1. Show how glucose can be converted to two equivalents of pyruvate. Include mechanisms for each of these reactions. See attached page 2. What role does the conversion of an aldose to a ketose play in the net glycolytic reaction scheme? This is necessary to prepare the hexose for the reverse aldol c ...
... 1. Show how glucose can be converted to two equivalents of pyruvate. Include mechanisms for each of these reactions. See attached page 2. What role does the conversion of an aldose to a ketose play in the net glycolytic reaction scheme? This is necessary to prepare the hexose for the reverse aldol c ...
Cell Respiration and Metabolism
... Units of Metabolic rate - Metabolic rate is measured as: Calories per square meter per hour (Calories/m2/hr) -m2 is the measure of body surface area. As an example BMR can be calculated from the amount of O2 consumption: A subject consumes 15 L of O2 in 1 hour at basal conditions, Caloric equivalen ...
... Units of Metabolic rate - Metabolic rate is measured as: Calories per square meter per hour (Calories/m2/hr) -m2 is the measure of body surface area. As an example BMR can be calculated from the amount of O2 consumption: A subject consumes 15 L of O2 in 1 hour at basal conditions, Caloric equivalen ...
P-glycoprotein Activation Monitored via ATP Hydrolysis and ATP
... We investigated the relationship between the rate of ATP hydrolysis and ATP synthesis upon P-glycoprotein activation for several structurally different drugs, including local anaesthetics, cyclic peptides, and cytotoxic drugs. ATP hydrolysis was assessed by spectroscopically monitoring the release o ...
... We investigated the relationship between the rate of ATP hydrolysis and ATP synthesis upon P-glycoprotein activation for several structurally different drugs, including local anaesthetics, cyclic peptides, and cytotoxic drugs. ATP hydrolysis was assessed by spectroscopically monitoring the release o ...
here - Sites@PSU
... Lactococcus sp. Lactobacillus sp. Leuconostoc sp. Pediococcus sp. Oenococcus sp. Streptococcus sp. Enterococcus sp. Sporolactobacillus sp. Carnobacterium sp. Aerococcus sp. Tetragenococcus sp. Vagococcus sp. Weisella sp. ...
... Lactococcus sp. Lactobacillus sp. Leuconostoc sp. Pediococcus sp. Oenococcus sp. Streptococcus sp. Enterococcus sp. Sporolactobacillus sp. Carnobacterium sp. Aerococcus sp. Tetragenococcus sp. Vagococcus sp. Weisella sp. ...
General Biology 115 Summer 2014
... Oxygen cannot be reduced Electrons cannot flow to Complex III Less H+ will be pumped across the inner mitochondrial membrane B&C A, B & C ...
... Oxygen cannot be reduced Electrons cannot flow to Complex III Less H+ will be pumped across the inner mitochondrial membrane B&C A, B & C ...
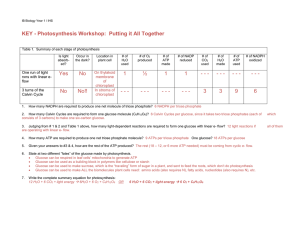
KEY - Photosynthesis Workshop: Putting it All Together
... 2. How many Calvin Cycles are required to form one glucose molecule (C6H12O6)? 6 Calvin Cycles per glucose, since it takes two triose phosphates (each of consists of 3 carbons) to make one six-carbon glucose. ...
... 2. How many Calvin Cycles are required to form one glucose molecule (C6H12O6)? 6 Calvin Cycles per glucose, since it takes two triose phosphates (each of consists of 3 carbons) to make one six-carbon glucose. ...
Midterm Review Project Ch 5
... primary structure: amino acid sequence; secondary structure: coils and folds as a result of hydrogen bonds, alpha- helix, beta-pleated sheet; tertiary structure: overall shape of polypeptide created by hydrophobic interaction ( hydrophobic side chains end up at core of protein) and disulfide bridges ...
... primary structure: amino acid sequence; secondary structure: coils and folds as a result of hydrogen bonds, alpha- helix, beta-pleated sheet; tertiary structure: overall shape of polypeptide created by hydrophobic interaction ( hydrophobic side chains end up at core of protein) and disulfide bridges ...
Lecture 13: Fighting Entropy II: Respiration
... cycle, NADH and FADH2 carry most of the energy extracted from food • These two molecules transport the high energy electrons generated by the breakdown of glucose to pyruvate (during glycolysis) and pyruvate to oxaloacetate and CO2 (during the citric acid cycle) and donate them to the electron trans ...
... cycle, NADH and FADH2 carry most of the energy extracted from food • These two molecules transport the high energy electrons generated by the breakdown of glucose to pyruvate (during glycolysis) and pyruvate to oxaloacetate and CO2 (during the citric acid cycle) and donate them to the electron trans ...
Hexokinase
... Figure 18.2 Pyruvate produced in glycolysis can be utilized by cells in several ways. In animals, pyruvate is normally converted to acetylcoenzyme A, which is then oxidized in the TCA cycle to produce CO2. When oxygen is limited, pyruvate can be converted to lactate. Alcoholic fermentation in yeast ...
... Figure 18.2 Pyruvate produced in glycolysis can be utilized by cells in several ways. In animals, pyruvate is normally converted to acetylcoenzyme A, which is then oxidized in the TCA cycle to produce CO2. When oxygen is limited, pyruvate can be converted to lactate. Alcoholic fermentation in yeast ...
Intermediary Metabolism - PBL-J-2015
... phosphate ester synthesis using the alcohol on the glucose and a phosphate from ATP. This first reaction is endothermic and thus requires energy from a coupled reaction with ATP. ATP is used by being hydrolyzed to ADP and phosphate giving off energy and the phosphate for reaction with the glucose fo ...
... phosphate ester synthesis using the alcohol on the glucose and a phosphate from ATP. This first reaction is endothermic and thus requires energy from a coupled reaction with ATP. ATP is used by being hydrolyzed to ADP and phosphate giving off energy and the phosphate for reaction with the glucose fo ...
"Central Pathways of Carbohydrate Metabolism". In: Microbial
... in lactic acid bacteria (Streptococcus, Lactococcus, Lactobacillus), pyruvate is reduced to lactate. Other microorganisms that use the EMP pathway have the capacity to convert pyruvate to a wide variety of other fermentation end products. These fermentation pathways are discussed in more detail in C ...
... in lactic acid bacteria (Streptococcus, Lactococcus, Lactobacillus), pyruvate is reduced to lactate. Other microorganisms that use the EMP pathway have the capacity to convert pyruvate to a wide variety of other fermentation end products. These fermentation pathways are discussed in more detail in C ...
Lesson
... ALCOHOL FERMENTATION Occurs in yeast, fungi, bacteria & plants Pyruvate becomes acetaldehyde Produces CO 2 ...
... ALCOHOL FERMENTATION Occurs in yeast, fungi, bacteria & plants Pyruvate becomes acetaldehyde Produces CO 2 ...
cannot
... Repetition of the Beta Oxidation Cycle The shortened fatty acyl-CoA that was the product of the last reaction now goes through another beta oxidation cycle. This keeps happening until eventually you wind up with two molecules of acetyl-CoA in the final step. This acetyl-CoA is then available to be ...
... Repetition of the Beta Oxidation Cycle The shortened fatty acyl-CoA that was the product of the last reaction now goes through another beta oxidation cycle. This keeps happening until eventually you wind up with two molecules of acetyl-CoA in the final step. This acetyl-CoA is then available to be ...
PRACTICE SET 6 - UC Davis Plant Sciences
... Unsaturations at odd carbons require only an isomerase, bypassing the first FADH2 synthesizing oxidation step. Unsaturations at an even C require both the 2,4-dienoylCoA reductase and an isomerase. This consumes one NADPH. Total 120 + 14 + 27 = 161-2 for activation = 159 ATP ...
... Unsaturations at odd carbons require only an isomerase, bypassing the first FADH2 synthesizing oxidation step. Unsaturations at an even C require both the 2,4-dienoylCoA reductase and an isomerase. This consumes one NADPH. Total 120 + 14 + 27 = 161-2 for activation = 159 ATP ...
Citric acid cycle
The citric acid cycle – also known as the tricarboxylic acid (TCA) cycle or the Krebs cycle – is a series of chemical reactions used by all aerobic organisms to generate energy through the oxidation of acetate derived from carbohydrates, fats and proteins into carbon dioxide and chemical energy in the form of adenosine triphosphate (ATP). In addition, the cycle provides precursors of certain amino acids as well as the reducing agent NADH that is used in numerous other biochemical reactions. Its central importance to many biochemical pathways suggests that it was one of the earliest established components of cellular metabolism and may have originated abiogenically.The name of this metabolic pathway is derived from citric acid (a type of tricarboxylic acid) that is consumed and then regenerated by this sequence of reactions to complete the cycle. In addition, the cycle consumes acetate (in the form of acetyl-CoA) and water, reduces NAD+ to NADH, and produces carbon dioxide as a waste byproduct. The NADH generated by the TCA cycle is fed into the oxidative phosphorylation (electron transport) pathway. The net result of these two closely linked pathways is the oxidation of nutrients to produce usable chemical energy in the form of ATP.In eukaryotic cells, the citric acid cycle occurs in the matrix of the mitochondrion. In prokaryotic cells, such as bacteria which lack mitochondria, the TCA reaction sequence is performed in the cytosol with the proton gradient for ATP production being across the cell's surface (plasma membrane) rather than the inner membrane of the mitochondrion.