Chemostat Design and Theory
... The People’s Chemostat – an EcLF Design The “People’s” chemostat considered here had its origins in 1973 at the University of Massachusetts, when Bruce Levin and Dennis Searcy constructed it to do low budget population dynamic and evolutionary studies with E. coli. (At the time, Bruce thought E. col ...
... The People’s Chemostat – an EcLF Design The “People’s” chemostat considered here had its origins in 1973 at the University of Massachusetts, when Bruce Levin and Dennis Searcy constructed it to do low budget population dynamic and evolutionary studies with E. coli. (At the time, Bruce thought E. col ...
respiration_DSE_revi..
... Coenzyme A (CoA) is a large molecule (and a vitamin) that acts as a coenzyme. The conversion of pyruvate to acetylCoA is an coupled oxidationreduction reaction in which high energy electrons are removed from pyruvate and end up in NADH. The three carbon pyruvate is split into CO2 and the two carbon ...
... Coenzyme A (CoA) is a large molecule (and a vitamin) that acts as a coenzyme. The conversion of pyruvate to acetylCoA is an coupled oxidationreduction reaction in which high energy electrons are removed from pyruvate and end up in NADH. The three carbon pyruvate is split into CO2 and the two carbon ...
Answers - U of L Class Index
... The cells in the liver, but not skeletal muscle, contain a phosphatase enzyme needed to convert glucose--phosphate to free glucose that can diffuse through cell membranes into the blood stream. Glucose-6-phosphate, which is the end product of glycogenolysis in muscle cells, cannot diffuse easily acr ...
... The cells in the liver, but not skeletal muscle, contain a phosphatase enzyme needed to convert glucose--phosphate to free glucose that can diffuse through cell membranes into the blood stream. Glucose-6-phosphate, which is the end product of glycogenolysis in muscle cells, cannot diffuse easily acr ...
Biosynthesis of Nucleotides Biosynthesis of Nucleotides
... Cytidine deaminase (activated by dCTP inhibited by dTTP) Of the 4 dNTPs, only dCTP does not interact with the regulatory sites on ribonucleotide reductase, instead it interacts with dCMP deaminase. ...
... Cytidine deaminase (activated by dCTP inhibited by dTTP) Of the 4 dNTPs, only dCTP does not interact with the regulatory sites on ribonucleotide reductase, instead it interacts with dCMP deaminase. ...
Practice Exam III answers
... 3). For the reaction catalyzed by adenylate kinase: ATP + AMP 2 ADP The overall G’ 0 even though the cellular [AMP], [ADP], and [ATP] are far away from their equilibrium values. What is an alternative explanation for why this reaction operates with a G’ 0? a). Adenylate kinase is altering t ...
... 3). For the reaction catalyzed by adenylate kinase: ATP + AMP 2 ADP The overall G’ 0 even though the cellular [AMP], [ADP], and [ATP] are far away from their equilibrium values. What is an alternative explanation for why this reaction operates with a G’ 0? a). Adenylate kinase is altering t ...
The b-oxidation pathway as an energy source
... from anaerobic bacteria which were phagocytosed by eukaryote cells at the time oxygen appeared on earth, Similarities between mitochondria and bacteria include the presence of: • cardiolipin •transporters • ribosomes • circular RNA and DNA Therefore mitochondria protein synthesis should be inhibited ...
... from anaerobic bacteria which were phagocytosed by eukaryote cells at the time oxygen appeared on earth, Similarities between mitochondria and bacteria include the presence of: • cardiolipin •transporters • ribosomes • circular RNA and DNA Therefore mitochondria protein synthesis should be inhibited ...
Mechanism of Thymidylate Synthase, Cont`d
... Dehydrogenase • GAPDH is one of the key enzymes for glycolysis, reversibly catalyzes the first glycolytic reaction to involve oxidation-reduction • It converts the glyceraldehyde-3-phosphate (G3P) into the high energy phosphate compound, 1,3 bisphosphoglycerate (BPG), using NAD+ as a cofactor • BPG ...
... Dehydrogenase • GAPDH is one of the key enzymes for glycolysis, reversibly catalyzes the first glycolytic reaction to involve oxidation-reduction • It converts the glyceraldehyde-3-phosphate (G3P) into the high energy phosphate compound, 1,3 bisphosphoglycerate (BPG), using NAD+ as a cofactor • BPG ...
Cofactors
... Homologous enzymes catalyze related reactions; this is how trp and his biosynthesis enzymes seem to have evolved Variant: recruit some enzymes from another pathway without duplicating the whole thing (example: ubiquitination) ...
... Homologous enzymes catalyze related reactions; this is how trp and his biosynthesis enzymes seem to have evolved Variant: recruit some enzymes from another pathway without duplicating the whole thing (example: ubiquitination) ...
A cofactor is a non-protein chemical compound that is
... coenzyme A, FAD, and NAD+. This common structure may reflect a common evolutionary origin as part of ribozymes in an ancient RNAworld. It has been suggested that the AMP part of the molecule can be considered a kind of "handle" by which the enzyme can "grasp" the coenzyme to switch it between differ ...
... coenzyme A, FAD, and NAD+. This common structure may reflect a common evolutionary origin as part of ribozymes in an ancient RNAworld. It has been suggested that the AMP part of the molecule can be considered a kind of "handle" by which the enzyme can "grasp" the coenzyme to switch it between differ ...
Glycolysis
... opening, isomerization via an enediolate intermediate, and then ring closure. A similar reaction catalyzed by Triosephosphate Isomerase will be presented in detail. ...
... opening, isomerization via an enediolate intermediate, and then ring closure. A similar reaction catalyzed by Triosephosphate Isomerase will be presented in detail. ...
Lecture-Lipid Metabolism - Creighton Chemistry Webserver
... Reverse of -oxidation, but enzymes and control are different -oxidation - mitochondria, FA biosynthesis - cytosol First committed step - acetyl CoA carboxylase ...
... Reverse of -oxidation, but enzymes and control are different -oxidation - mitochondria, FA biosynthesis - cytosol First committed step - acetyl CoA carboxylase ...
Document
... insulin does not enter cells—it (like glucagon) binds to its receptor and various signals are transmitted into the cell (i.e., signal transduction), which results in various responses (induction of genes; stimulation of enzymes; translocation of GLUT4); some of these responses occur in all cells, so ...
... insulin does not enter cells—it (like glucagon) binds to its receptor and various signals are transmitted into the cell (i.e., signal transduction), which results in various responses (induction of genes; stimulation of enzymes; translocation of GLUT4); some of these responses occur in all cells, so ...
Biochemistry - Text of NPTEL IIT Video Lectures
... that we have gone through that is glycolysis for acetyl coenzyme A production. We have already seen the breakdown of glucose to pyruvate and we will see now how pyruvate actually gets to acetyl coenzyme A that then gets into the tricarboxylic acid cycle which eventually leads to the production of ca ...
... that we have gone through that is glycolysis for acetyl coenzyme A production. We have already seen the breakdown of glucose to pyruvate and we will see now how pyruvate actually gets to acetyl coenzyme A that then gets into the tricarboxylic acid cycle which eventually leads to the production of ca ...
Honors Chemistry: Ch. 12 – Stoichiometry Some useful terms
... 4.) Calculate the mass of silver needed to react with chlorine to produce 84 g of silver chloride (Hint: Write a balanced equation first). 5.) Calculate the number of liters of oxygen gas needed to produce 15.0 liters of dinitrogen trioxide. Assume all gases are at STP. 2N2(g) + 3O2(g) 2N2O3(g) 6. ...
... 4.) Calculate the mass of silver needed to react with chlorine to produce 84 g of silver chloride (Hint: Write a balanced equation first). 5.) Calculate the number of liters of oxygen gas needed to produce 15.0 liters of dinitrogen trioxide. Assume all gases are at STP. 2N2(g) + 3O2(g) 2N2O3(g) 6. ...
PDF
... Maris et al., 2004b). The high intracellular pH ensures that the majority of lactic acid is present as the lactate anion, which is incapable of diffusing across the plasma membrane. Therefore, analogous to the export of other weak organic acid anions (Piper et al., 1998; Fernandes et al., 2005), it ...
... Maris et al., 2004b). The high intracellular pH ensures that the majority of lactic acid is present as the lactate anion, which is incapable of diffusing across the plasma membrane. Therefore, analogous to the export of other weak organic acid anions (Piper et al., 1998; Fernandes et al., 2005), it ...
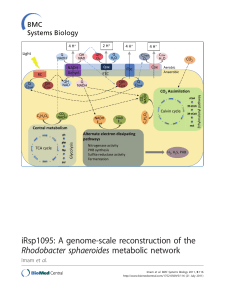
iRsp1095: A genome-scale reconstruction of the Rhodobacter
... To fill this knowledge gap, we are modeling the flow of carbon and reducing power in the well-studied photosynthetic bacterium Rhodobacter sphaeroides. This facultative bacterium is capable of either aerobic or anaerobic respiration, depending on the availability of oxygen (O2) or alternative electr ...
... To fill this knowledge gap, we are modeling the flow of carbon and reducing power in the well-studied photosynthetic bacterium Rhodobacter sphaeroides. This facultative bacterium is capable of either aerobic or anaerobic respiration, depending on the availability of oxygen (O2) or alternative electr ...
Metabolism, Lectures 25-27 Quadrant – 2 - vtu-nptel
... b) oxygen-forming photosynthesis c) the degradation of organic molecules with the released energy stored in ATP d) anaerobic respiration 4. The First Law of Thermodynamics states that energy can be a) created b) destroyed c) converted d) all of the above 5. The universal energy currency for all cell ...
... b) oxygen-forming photosynthesis c) the degradation of organic molecules with the released energy stored in ATP d) anaerobic respiration 4. The First Law of Thermodynamics states that energy can be a) created b) destroyed c) converted d) all of the above 5. The universal energy currency for all cell ...
Nutrient Cycles
... Nitrogen-containing substances such as ammonia (NH3), nitrate ions (NO3), and nitrite ions (NO2) are found in soil, in the wastes produced by many organisms, and in dead and decaying organic matter. ...
... Nitrogen-containing substances such as ammonia (NH3), nitrate ions (NO3), and nitrite ions (NO2) are found in soil, in the wastes produced by many organisms, and in dead and decaying organic matter. ...
Fundamentals: Bioenergetics and Enzyme Function
... 16. It is generally recognized the more ATP per liter of oxygen is produced from CHO than FFA. Evaluate the two pathways of catabolism and try to explain why this is so (a tough one !!!). 17. There are several potential rate-limiting reactions to FFA catabolism. Given a diagram of FFA catabolism, wh ...
... 16. It is generally recognized the more ATP per liter of oxygen is produced from CHO than FFA. Evaluate the two pathways of catabolism and try to explain why this is so (a tough one !!!). 17. There are several potential rate-limiting reactions to FFA catabolism. Given a diagram of FFA catabolism, wh ...
Microbial metabolism
Microbial metabolism is the means by which a microbe obtains the energy and nutrients (e.g. carbon) it needs to live and reproduce. Microbes use many different types of metabolic strategies and species can often be differentiated from each other based on metabolic characteristics. The specific metabolic properties of a microbe are the major factors in determining that microbe’s ecological niche, and often allow for that microbe to be useful in industrial processes or responsible for biogeochemical cycles.== Types of microbial metabolism ==All microbial metabolisms can be arranged according to three principles:1. How the organism obtains carbon for synthesising cell mass: autotrophic – carbon is obtained from carbon dioxide (CO2) heterotrophic – carbon is obtained from organic compounds mixotrophic – carbon is obtained from both organic compounds and by fixing carbon dioxide2. How the organism obtains reducing equivalents used either in energy conservation or in biosynthetic reactions: lithotrophic – reducing equivalents are obtained from inorganic compounds organotrophic – reducing equivalents are obtained from organic compounds3. How the organism obtains energy for living and growing: chemotrophic – energy is obtained from external chemical compounds phototrophic – energy is obtained from lightIn practice, these terms are almost freely combined. Typical examples are as follows: chemolithoautotrophs obtain energy from the oxidation of inorganic compounds and carbon from the fixation of carbon dioxide. Examples: Nitrifying bacteria, Sulfur-oxidizing bacteria, Iron-oxidizing bacteria, Knallgas-bacteria photolithoautotrophs obtain energy from light and carbon from the fixation of carbon dioxide, using reducing equivalents from inorganic compounds. Examples: Cyanobacteria (water (H2O) as reducing equivalent donor), Chlorobiaceae, Chromatiaceae (hydrogen sulfide (H2S) as reducing equivalent donor), Chloroflexus (hydrogen (H2) as reducing equivalent donor) chemolithoheterotrophs obtain energy from the oxidation of inorganic compounds, but cannot fix carbon dioxide (CO2). Examples: some Thiobacilus, some Beggiatoa, some Nitrobacter spp., Wolinella (with H2 as reducing equivalent donor), some Knallgas-bacteria, some sulfate-reducing bacteria chemoorganoheterotrophs obtain energy, carbon, and reducing equivalents for biosynthetic reactions from organic compounds. Examples: most bacteria, e. g. Escherichia coli, Bacillus spp., Actinobacteria photoorganoheterotrophs obtain energy from light, carbon and reducing equivalents for biosynthetic reactions from organic compounds. Some species are strictly heterotrophic, many others can also fix carbon dioxide and are mixotrophic. Examples: Rhodobacter, Rhodopseudomonas, Rhodospirillum, Rhodomicrobium, Rhodocyclus, Heliobacterium, Chloroflexus (alternatively to photolithoautotrophy with hydrogen)