Surprising variety in energy metabolism within Trypanosomatidae
... trypanosomatids (see Figures 1 and 2). Excretion of pyruvate as the main end product is highly unusual, even among trypanosomatids. In most eukaryotes, pyruvate is transported into the mitochondria for further oxidation and the accompanying ATP formation. If the use of the mitochondrial respiratory ...
... trypanosomatids (see Figures 1 and 2). Excretion of pyruvate as the main end product is highly unusual, even among trypanosomatids. In most eukaryotes, pyruvate is transported into the mitochondria for further oxidation and the accompanying ATP formation. If the use of the mitochondrial respiratory ...
chm 205 - National Open University of Nigeria
... Amongst all elements of this group, carbon is the only one to occur in the elemental state as diamond and graphite. These are the two naturally occurring allotropic forms of carbon. As stated above, carbon, in the combined form, is an essential constituent of all living systems; you will study about ...
... Amongst all elements of this group, carbon is the only one to occur in the elemental state as diamond and graphite. These are the two naturally occurring allotropic forms of carbon. As stated above, carbon, in the combined form, is an essential constituent of all living systems; you will study about ...
Metabolic Activity of Heterotrophic Bacteria in the Presence of Humic
... the investigated bacteria when accompanied by various fractions of humic substances. The data provide information on the Jeziorak bacteria being most active in using the humin acids isolated from bottom sediments, while humin acids isolated from water were most attractive for the lake Jasne bacteria ...
... the investigated bacteria when accompanied by various fractions of humic substances. The data provide information on the Jeziorak bacteria being most active in using the humin acids isolated from bottom sediments, while humin acids isolated from water were most attractive for the lake Jasne bacteria ...
Test 5 Ch 2 - Kenton County Schools
... ____ 11. Refer to the illustration above. Which of the following statements regarding the graph is true? a. Reaction 2 occurs faster than Reaction 3 because Reaction 2 requires more energy than Reaction 3. b. The difference between the graphs shown for Reaction 2 and Reaction 3 occurs because of a ...
... ____ 11. Refer to the illustration above. Which of the following statements regarding the graph is true? a. Reaction 2 occurs faster than Reaction 3 because Reaction 2 requires more energy than Reaction 3. b. The difference between the graphs shown for Reaction 2 and Reaction 3 occurs because of a ...
1. Products of Amino Acid Transamination Name
... (c) Phenylalanine is converted to phenylpyruvate by transamination, a reaction that has an equilibrium constant of about 1.0. Phenyllactate is formed from phenylpyruvate by reduction (see Fig. 18–25). This pathway is of importance only when phenylalanine hydroxylase is defective. (d) The normal cat ...
... (c) Phenylalanine is converted to phenylpyruvate by transamination, a reaction that has an equilibrium constant of about 1.0. Phenyllactate is formed from phenylpyruvate by reduction (see Fig. 18–25). This pathway is of importance only when phenylalanine hydroxylase is defective. (d) The normal cat ...
Outline
... reduction half-reactions. 2 Describe how a cell can be constructed using a redox reaction in which halfreactions are contained in half-cells joined by a salt bridge or separated by a ...
... reduction half-reactions. 2 Describe how a cell can be constructed using a redox reaction in which halfreactions are contained in half-cells joined by a salt bridge or separated by a ...
The Cardiovascular System and Exercise
... A technique for measuring body composition has been developed using the same principles as under water weighing. The technique uses air, as opposed to water and is known as air displacement plethysmography (ADP). Subjects enter a sealed chamber that measures their body volume through the displacemen ...
... A technique for measuring body composition has been developed using the same principles as under water weighing. The technique uses air, as opposed to water and is known as air displacement plethysmography (ADP). Subjects enter a sealed chamber that measures their body volume through the displacemen ...
S. Salgueiro Machado • M. A. ... J. P. van Dijken • ...
... medium supplemented with ethanol, L-lactate or acetate. Nevertheless, biomass yields were extremely low in comparison to values reported for other bacteria. Cells exhibited high oxidation rates with ethanol and racemic glycidol (2,3-epoxy-l-propanol). Ethanol- and glycidol-dependent oxygen-uptake ca ...
... medium supplemented with ethanol, L-lactate or acetate. Nevertheless, biomass yields were extremely low in comparison to values reported for other bacteria. Cells exhibited high oxidation rates with ethanol and racemic glycidol (2,3-epoxy-l-propanol). Ethanol- and glycidol-dependent oxygen-uptake ca ...
An Introduction to Metabolism
... resources of the cell. Some metabolic pathways release energy by breaking down complex molecules to simpler compounds. These degradative processes are called catabolic pathways, or breakdown pathways. A major pathway of catabolism is cellular respiration, in which the sugar glucose and other organic ...
... resources of the cell. Some metabolic pathways release energy by breaking down complex molecules to simpler compounds. These degradative processes are called catabolic pathways, or breakdown pathways. A major pathway of catabolism is cellular respiration, in which the sugar glucose and other organic ...
Biology`s Gasoline: Oxidation of Fatty Acids Fats: our unpopular best
... Now here’s the cool, or at least the easy, thing. Since we ended up with a new acyl-CoA (two carbons shorter than the original one), we can do the same thing again to this smaller substrate with the exact same reactions. This happens again and again until the original fatty acid is “whittled down” t ...
... Now here’s the cool, or at least the easy, thing. Since we ended up with a new acyl-CoA (two carbons shorter than the original one), we can do the same thing again to this smaller substrate with the exact same reactions. This happens again and again until the original fatty acid is “whittled down” t ...
SP10 - Miss S. Harvey
... Producers The producers or autotrophs (Figure 1) are organisms that make their own food, usually using energy from the Sun in a process called photosynthesis. You will learn more about this process in Chapter 4. Producers are also an important food source for other organisms. Almost all plants can p ...
... Producers The producers or autotrophs (Figure 1) are organisms that make their own food, usually using energy from the Sun in a process called photosynthesis. You will learn more about this process in Chapter 4. Producers are also an important food source for other organisms. Almost all plants can p ...
Second
... A student just started to study protein translocation with the eventual goal of isolating the individual components involved. His first task was to isolate the different subcellular fractions that are required for this process. Unfortunately, his labels fell off and he could not tell which fraction ...
... A student just started to study protein translocation with the eventual goal of isolating the individual components involved. His first task was to isolate the different subcellular fractions that are required for this process. Unfortunately, his labels fell off and he could not tell which fraction ...
Ass3_ans - The University of Sydney
... To provide reducing equivalents To mop up excess NADPH Oxygen gas isn't used in the grand scheme, it's just required here to make a balanced equation It reflects the consumption of oxygen at the end of the electron transport chain To enable carbon dioxide formation ...
... To provide reducing equivalents To mop up excess NADPH Oxygen gas isn't used in the grand scheme, it's just required here to make a balanced equation It reflects the consumption of oxygen at the end of the electron transport chain To enable carbon dioxide formation ...
HORIZONS Modelling emergent trophic strategies in plankton
... are respiratory costs associated with the actual functions proportional to the uptakes of carbon, nutrients and other organisms. A central “alternative cost” is that allocation to one trait comes at the expense of allocation to other, represented by the allocation-triangle in Fig. 1. The trade-offs ...
... are respiratory costs associated with the actual functions proportional to the uptakes of carbon, nutrients and other organisms. A central “alternative cost” is that allocation to one trait comes at the expense of allocation to other, represented by the allocation-triangle in Fig. 1. The trade-offs ...
Ass3 - The University of Sydney
... The fate of 5-carbon sugar phosphates in the pentose phosphate pathway The reactions of glycolysis The trapping of glucose ...
... The fate of 5-carbon sugar phosphates in the pentose phosphate pathway The reactions of glycolysis The trapping of glucose ...
Chemical Biology - Chem 370 (3 credits)
... will focus on structure and function of the chemistry of biological systems while the second half of the course will concentrate on metabolic cycles and energy production. This course is designed to extend the chemical reactions and mechanisms studied in undergraduate organic chemistry to biological ...
... will focus on structure and function of the chemistry of biological systems while the second half of the course will concentrate on metabolic cycles and energy production. This course is designed to extend the chemical reactions and mechanisms studied in undergraduate organic chemistry to biological ...
How Cells Obtain Energy
... use energy. Nutrients and other molecules are imported into the cell, metabolized (broken down) and possibly synthesized into new molecules, modified if needed, transported around the cell, and possibly distributed to the entire organism. For example, the large proteins that make up muscles are buil ...
... use energy. Nutrients and other molecules are imported into the cell, metabolized (broken down) and possibly synthesized into new molecules, modified if needed, transported around the cell, and possibly distributed to the entire organism. For example, the large proteins that make up muscles are buil ...
Biochemical Characteristics and Viability of Probiotic and Yogurt
... (Lactobacillus acidophilus LA-5 and Bifidobacterium lactis BB-12) and yogurt bacteria (Streptococcus thermophilus and Lactobacillus delbrueckii ssp. bulgaricus) in yogurt during the fermentation, immediately after fermentation and during refrigerated storage (21 d, 4˚C). Also the biochemical charact ...
... (Lactobacillus acidophilus LA-5 and Bifidobacterium lactis BB-12) and yogurt bacteria (Streptococcus thermophilus and Lactobacillus delbrueckii ssp. bulgaricus) in yogurt during the fermentation, immediately after fermentation and during refrigerated storage (21 d, 4˚C). Also the biochemical charact ...
Vitamins and Coenzymes - KSU - Home
... Structure of β-Carbonic Anhydrase • Found in plans which is an evolutionarily distinct enzyme but participates in the same reaction and also uses a Zn2+ in its active site • It helps raise the concentration of CO2 within the chloroplast to increase the carboxylation rate of the enzyme Rubisco • It ...
... Structure of β-Carbonic Anhydrase • Found in plans which is an evolutionarily distinct enzyme but participates in the same reaction and also uses a Zn2+ in its active site • It helps raise the concentration of CO2 within the chloroplast to increase the carboxylation rate of the enzyme Rubisco • It ...
11. PHOTOSYNTHETIC PATHWAYS - Development of e
... he pyruvic acid may be oxidized to CO2 by the pathway of Kreb’s cycle or it may be reconverted to phosphoenol pyruvic acid and synthesize sugar by C3 cycle. ...
... he pyruvic acid may be oxidized to CO2 by the pathway of Kreb’s cycle or it may be reconverted to phosphoenol pyruvic acid and synthesize sugar by C3 cycle. ...
Nucleotide Metabolism Nucleotide sources - Rose
... The first reaction of the purine synthesis pathway, catalyzed by ribose-5phosphate pyrophosphokinase, produces phosphoribosylpyrophosphate (PRPP). PRPP is not committed to purine biosynthesis; it is also used for other processes (such as pyrimidine biosynthesis, histidine biosynthesis, and nucleotid ...
... The first reaction of the purine synthesis pathway, catalyzed by ribose-5phosphate pyrophosphokinase, produces phosphoribosylpyrophosphate (PRPP). PRPP is not committed to purine biosynthesis; it is also used for other processes (such as pyrimidine biosynthesis, histidine biosynthesis, and nucleotid ...
Citric acid cycle - Imperial College London
... converted into acetyl-CoA by decarboxylation and enters the citric acid cycle. In protein catabolism, proteins are broken down by proteases into their constituent amino acids. The carbon backbone of these amino acids can become a source of energy by being converted to acetyl-CoA and entering into th ...
... converted into acetyl-CoA by decarboxylation and enters the citric acid cycle. In protein catabolism, proteins are broken down by proteases into their constituent amino acids. The carbon backbone of these amino acids can become a source of energy by being converted to acetyl-CoA and entering into th ...
CHAPTER 6
... liberated in this oxidation flow through the electrontransport chain and drive the synthesis of ATP in oxidative phosphorylation. In eukaryotic cells, this overall process occurs in mitochondria. ...
... liberated in this oxidation flow through the electrontransport chain and drive the synthesis of ATP in oxidative phosphorylation. In eukaryotic cells, this overall process occurs in mitochondria. ...
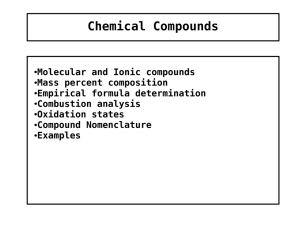
Chemical Compounds
... 4. The oxidation state of hydrogen is generally +1 except when it is bonded to metals such as sodium (NaH) in which case it's oxidation number is -1. 5. Fluorine has an oxidation number of -1 in its compounds … always. Group 1 elements have an oxidation number of +1 in their compounds … always. Grou ...
... 4. The oxidation state of hydrogen is generally +1 except when it is bonded to metals such as sodium (NaH) in which case it's oxidation number is -1. 5. Fluorine has an oxidation number of -1 in its compounds … always. Group 1 elements have an oxidation number of +1 in their compounds … always. Grou ...
Microbial metabolism
Microbial metabolism is the means by which a microbe obtains the energy and nutrients (e.g. carbon) it needs to live and reproduce. Microbes use many different types of metabolic strategies and species can often be differentiated from each other based on metabolic characteristics. The specific metabolic properties of a microbe are the major factors in determining that microbe’s ecological niche, and often allow for that microbe to be useful in industrial processes or responsible for biogeochemical cycles.== Types of microbial metabolism ==All microbial metabolisms can be arranged according to three principles:1. How the organism obtains carbon for synthesising cell mass: autotrophic – carbon is obtained from carbon dioxide (CO2) heterotrophic – carbon is obtained from organic compounds mixotrophic – carbon is obtained from both organic compounds and by fixing carbon dioxide2. How the organism obtains reducing equivalents used either in energy conservation or in biosynthetic reactions: lithotrophic – reducing equivalents are obtained from inorganic compounds organotrophic – reducing equivalents are obtained from organic compounds3. How the organism obtains energy for living and growing: chemotrophic – energy is obtained from external chemical compounds phototrophic – energy is obtained from lightIn practice, these terms are almost freely combined. Typical examples are as follows: chemolithoautotrophs obtain energy from the oxidation of inorganic compounds and carbon from the fixation of carbon dioxide. Examples: Nitrifying bacteria, Sulfur-oxidizing bacteria, Iron-oxidizing bacteria, Knallgas-bacteria photolithoautotrophs obtain energy from light and carbon from the fixation of carbon dioxide, using reducing equivalents from inorganic compounds. Examples: Cyanobacteria (water (H2O) as reducing equivalent donor), Chlorobiaceae, Chromatiaceae (hydrogen sulfide (H2S) as reducing equivalent donor), Chloroflexus (hydrogen (H2) as reducing equivalent donor) chemolithoheterotrophs obtain energy from the oxidation of inorganic compounds, but cannot fix carbon dioxide (CO2). Examples: some Thiobacilus, some Beggiatoa, some Nitrobacter spp., Wolinella (with H2 as reducing equivalent donor), some Knallgas-bacteria, some sulfate-reducing bacteria chemoorganoheterotrophs obtain energy, carbon, and reducing equivalents for biosynthetic reactions from organic compounds. Examples: most bacteria, e. g. Escherichia coli, Bacillus spp., Actinobacteria photoorganoheterotrophs obtain energy from light, carbon and reducing equivalents for biosynthetic reactions from organic compounds. Some species are strictly heterotrophic, many others can also fix carbon dioxide and are mixotrophic. Examples: Rhodobacter, Rhodopseudomonas, Rhodospirillum, Rhodomicrobium, Rhodocyclus, Heliobacterium, Chloroflexus (alternatively to photolithoautotrophy with hydrogen)