The Citric Acid Cycle
... There is no net conversion of acetate (also from fatty acids and amino acids) to any of the citric acid cycle intermediate, thus neither to carbohydrates. Net conversion of acetate to four-carbon citric acid cycle intermediates occurs via the glyoxylate cycle, occurring in plants, certain inverteb ...
... There is no net conversion of acetate (also from fatty acids and amino acids) to any of the citric acid cycle intermediate, thus neither to carbohydrates. Net conversion of acetate to four-carbon citric acid cycle intermediates occurs via the glyoxylate cycle, occurring in plants, certain inverteb ...
Answers - logo Pre-U Chemistry Textbook
... ∆rH = –577 kJ mol–1, which is highly exothermic so gases produced will expand rapidly at the high temperatures. Also, there are __ 32 more moles of products than reactants. ...
... ∆rH = –577 kJ mol–1, which is highly exothermic so gases produced will expand rapidly at the high temperatures. Also, there are __ 32 more moles of products than reactants. ...
Teaching Guide - BioMEDIA Associates
... Regulation and Behavior Behavior is one kind of response an organism can make to an internal or environmental stimulus. A behavioral response requires coordination and communication at many levels, including cells, organ systems, and whole organisms. Behavioral response is a set of actions determine ...
... Regulation and Behavior Behavior is one kind of response an organism can make to an internal or environmental stimulus. A behavioral response requires coordination and communication at many levels, including cells, organ systems, and whole organisms. Behavioral response is a set of actions determine ...
db02-8
... Round 8–Questions by CB with science help by Seth Teitler and DePauw QB 1) It is most easily made with eggs that are three or four days old, and refrigerated eggs are easier to use as they separate from the yolk better. The final product is hygroscopic, meaning it attracts moisture, so its best to m ...
... Round 8–Questions by CB with science help by Seth Teitler and DePauw QB 1) It is most easily made with eggs that are three or four days old, and refrigerated eggs are easier to use as they separate from the yolk better. The final product is hygroscopic, meaning it attracts moisture, so its best to m ...
Class-XII, Summer assignment
... Ans: In H3PO2, two H atoms are bonded directly to P atom which imparts Reducing character to the acid. 20. What is the basicity of H3PO4? Ans: Three P–OH groups are present in the molecule of H3PO4. Therefore, its basicity is three. 21. Phosphorous in solid state is ionic, why? Ans: In the solid sta ...
... Ans: In H3PO2, two H atoms are bonded directly to P atom which imparts Reducing character to the acid. 20. What is the basicity of H3PO4? Ans: Three P–OH groups are present in the molecule of H3PO4. Therefore, its basicity is three. 21. Phosphorous in solid state is ionic, why? Ans: In the solid sta ...
The Inherited Metabolic Disorders News
... PDCD is a rare disorder. Pyruvate dehydrogenase (PDH) is an essential and important enzyme in the mitochondrial metabolism. The effects of this deficiency are serious as there is a lack of energy production and build up of lactic acid. PDH catalyzes the oxidation of pyruvate to acetyl CoA (Fig 1). W ...
... PDCD is a rare disorder. Pyruvate dehydrogenase (PDH) is an essential and important enzyme in the mitochondrial metabolism. The effects of this deficiency are serious as there is a lack of energy production and build up of lactic acid. PDH catalyzes the oxidation of pyruvate to acetyl CoA (Fig 1). W ...
Succinate Dehydrogenase of Saccharomyces cerevisiae
... produced. Pyruvate may be regarded as the preliminary final product of the degradation in a strictly formal sense - because it is here that the pathway ramifies: pyruvate is hydrated under anaerobic conditions resulting in either lactate (in lactic-acid bacteria) or ethanol (in yeast). If glycolysis ...
... produced. Pyruvate may be regarded as the preliminary final product of the degradation in a strictly formal sense - because it is here that the pathway ramifies: pyruvate is hydrated under anaerobic conditions resulting in either lactate (in lactic-acid bacteria) or ethanol (in yeast). If glycolysis ...
Facultative Anaerobiosis in the Invertebrates: Pathways and Control
... helminths studied to date, regardless of their oxygen requirements, be they aerobic or anaerobic, are capable of the complete oxidation of substrates to CO2 and water. All of those examined accumulate organic end-products. Since substrates are not completely metabolized, it would appear that termina ...
... helminths studied to date, regardless of their oxygen requirements, be they aerobic or anaerobic, are capable of the complete oxidation of substrates to CO2 and water. All of those examined accumulate organic end-products. Since substrates are not completely metabolized, it would appear that termina ...
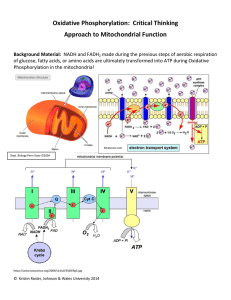
practice oxidative phosphorylation worksheet11
... A series of proteins in the inner membrane will be used to do so. Active transport utilized to move H+ against concentration gradient in the Electron Transport Chain! A series of redox reactions are employed as H+ ions are moved against a concentration gradient via active transport. Protein Complexe ...
... A series of proteins in the inner membrane will be used to do so. Active transport utilized to move H+ against concentration gradient in the Electron Transport Chain! A series of redox reactions are employed as H+ ions are moved against a concentration gradient via active transport. Protein Complexe ...
Prediction of Maximum Yields of Metabolites and Optimal Pathways
... for the intracellular reactions along with mass balances around the intracellular metabolites. In this study, metabolic flux analyses were carried out to estimate flux distributions for the maximum in silico yields of various metabolites in Escherichia coli. The maximum in silico yields of acetic ac ...
... for the intracellular reactions along with mass balances around the intracellular metabolites. In this study, metabolic flux analyses were carried out to estimate flux distributions for the maximum in silico yields of various metabolites in Escherichia coli. The maximum in silico yields of acetic ac ...
The Science of Onion Flavor Onions are one of the most widely used
... flavor they add to cooked vegetable and meat dishes. But pick up a fresh onion and what do you smell? Nothing! That’s because, like many vegetables, onions do not develop their characteristic flavor until their cells are damaged by cutting, slicing, chopping, or cooking. Now the strong pungent smell ...
... flavor they add to cooked vegetable and meat dishes. But pick up a fresh onion and what do you smell? Nothing! That’s because, like many vegetables, onions do not develop their characteristic flavor until their cells are damaged by cutting, slicing, chopping, or cooking. Now the strong pungent smell ...
Major players on the microbial stage: why archaea
... eukarya and archaea is widely accepted, the origin of the Domain Eukarya is one of the most controversial areas of evolutionary biology (Embley & Martin, 2006). Variations of two broad theories on the origin of eukaryotic cells involving the Archaea have been presented. The first posits that the Arc ...
... eukarya and archaea is widely accepted, the origin of the Domain Eukarya is one of the most controversial areas of evolutionary biology (Embley & Martin, 2006). Variations of two broad theories on the origin of eukaryotic cells involving the Archaea have been presented. The first posits that the Arc ...
Carbohydrate Metabolism
... as structural elements in living cells. This chapter looks at the role of carbohydrates in energy production. Because the monosaccharide glucose is a prominent energy source in almost all living cells, major emphasis is placed on its synthesis, degradation, and storage. iving cells are in a state of ...
... as structural elements in living cells. This chapter looks at the role of carbohydrates in energy production. Because the monosaccharide glucose is a prominent energy source in almost all living cells, major emphasis is placed on its synthesis, degradation, and storage. iving cells are in a state of ...
Major contribution of both zooplankton and protists to the top
... 2015). Two possible hypotheses may explain their low in situ abundances: either light-derived energy has little effect on the growth and competitiveness of AAP, or, if there is an effect, there are other factors, unrelated to phototrophy, that may limit the ecological success of AAP bacteria more th ...
... 2015). Two possible hypotheses may explain their low in situ abundances: either light-derived energy has little effect on the growth and competitiveness of AAP, or, if there is an effect, there are other factors, unrelated to phototrophy, that may limit the ecological success of AAP bacteria more th ...
BI25M1
... It occurs in: no eukaryotes; a few prokaryotes, including Cyanobacteria; a few free-living bacteria; Rhizobium bacteroids living symbiotically in root cells of legumes. ...
... It occurs in: no eukaryotes; a few prokaryotes, including Cyanobacteria; a few free-living bacteria; Rhizobium bacteroids living symbiotically in root cells of legumes. ...
PURINE Lacture
... 1. Negative regulation of PRPP Synthatase & PRPP Amidotransferase is lost 2. PRPP levels are increased because of defects in salvage pathways Therefore, there is net increase in biosynthetic/degradation pathways!! ...
... 1. Negative regulation of PRPP Synthatase & PRPP Amidotransferase is lost 2. PRPP levels are increased because of defects in salvage pathways Therefore, there is net increase in biosynthetic/degradation pathways!! ...
INTERMEDIARY METABOLISM
... In addition to these major bases, a number of other purine and pyrimidine derivatives have been isolated in small amount from various nucleic acids. The transfer RNAs (t-RNAs) contain several unusual bases, such as methylated or dimethylated derivatives of adenine, guanine, cytosine and uracil. Othe ...
... In addition to these major bases, a number of other purine and pyrimidine derivatives have been isolated in small amount from various nucleic acids. The transfer RNAs (t-RNAs) contain several unusual bases, such as methylated or dimethylated derivatives of adenine, guanine, cytosine and uracil. Othe ...
Pacing Guide
... Fermentation reactions (lactic acid fermentation and ethyl alcohol fermentation), Role of accessory pigments and evidence for their existence, Aspects of Autumn coloration in trees, Leaf cross-sectional anatomy and function of these structures, Light dependent reactions and role of reactants and pro ...
... Fermentation reactions (lactic acid fermentation and ethyl alcohol fermentation), Role of accessory pigments and evidence for their existence, Aspects of Autumn coloration in trees, Leaf cross-sectional anatomy and function of these structures, Light dependent reactions and role of reactants and pro ...
Chapter 8: Energy generation:glycolysis
... proteins. Some enzyme cofactors are also activated carrier molecules. These include NAD+ and NADP+, each of which can carry energy in the form of a pair of electrons and a proton (H+ ion), converting the molecules into their reduced forms referred to as NADH and NADPH. The chemical equations for red ...
... proteins. Some enzyme cofactors are also activated carrier molecules. These include NAD+ and NADP+, each of which can carry energy in the form of a pair of electrons and a proton (H+ ion), converting the molecules into their reduced forms referred to as NADH and NADPH. The chemical equations for red ...
Fructose-1,6 - LSU School of Medicine
... Phosphoenolpyruvate carboxykinase is regulated at the level of ...
... Phosphoenolpyruvate carboxykinase is regulated at the level of ...
Microbial metabolism
Microbial metabolism is the means by which a microbe obtains the energy and nutrients (e.g. carbon) it needs to live and reproduce. Microbes use many different types of metabolic strategies and species can often be differentiated from each other based on metabolic characteristics. The specific metabolic properties of a microbe are the major factors in determining that microbe’s ecological niche, and often allow for that microbe to be useful in industrial processes or responsible for biogeochemical cycles.== Types of microbial metabolism ==All microbial metabolisms can be arranged according to three principles:1. How the organism obtains carbon for synthesising cell mass: autotrophic – carbon is obtained from carbon dioxide (CO2) heterotrophic – carbon is obtained from organic compounds mixotrophic – carbon is obtained from both organic compounds and by fixing carbon dioxide2. How the organism obtains reducing equivalents used either in energy conservation or in biosynthetic reactions: lithotrophic – reducing equivalents are obtained from inorganic compounds organotrophic – reducing equivalents are obtained from organic compounds3. How the organism obtains energy for living and growing: chemotrophic – energy is obtained from external chemical compounds phototrophic – energy is obtained from lightIn practice, these terms are almost freely combined. Typical examples are as follows: chemolithoautotrophs obtain energy from the oxidation of inorganic compounds and carbon from the fixation of carbon dioxide. Examples: Nitrifying bacteria, Sulfur-oxidizing bacteria, Iron-oxidizing bacteria, Knallgas-bacteria photolithoautotrophs obtain energy from light and carbon from the fixation of carbon dioxide, using reducing equivalents from inorganic compounds. Examples: Cyanobacteria (water (H2O) as reducing equivalent donor), Chlorobiaceae, Chromatiaceae (hydrogen sulfide (H2S) as reducing equivalent donor), Chloroflexus (hydrogen (H2) as reducing equivalent donor) chemolithoheterotrophs obtain energy from the oxidation of inorganic compounds, but cannot fix carbon dioxide (CO2). Examples: some Thiobacilus, some Beggiatoa, some Nitrobacter spp., Wolinella (with H2 as reducing equivalent donor), some Knallgas-bacteria, some sulfate-reducing bacteria chemoorganoheterotrophs obtain energy, carbon, and reducing equivalents for biosynthetic reactions from organic compounds. Examples: most bacteria, e. g. Escherichia coli, Bacillus spp., Actinobacteria photoorganoheterotrophs obtain energy from light, carbon and reducing equivalents for biosynthetic reactions from organic compounds. Some species are strictly heterotrophic, many others can also fix carbon dioxide and are mixotrophic. Examples: Rhodobacter, Rhodopseudomonas, Rhodospirillum, Rhodomicrobium, Rhodocyclus, Heliobacterium, Chloroflexus (alternatively to photolithoautotrophy with hydrogen)