Fatty Acid Catabolism Caloric Value of Fats and Carbohydrates
... Reactions of β-oxidation 1. Dehydrogenation of fatty acyl CoA to produce a trans double bond between the α and β carbons (or C-2 & C-3). the product is trans-∆2-enoyl-CoA reaction catalyzed by acyl CoA dehydrogenase. the electron acceptor is FAD the reaction is analogous to succinate dehydrogenase 2 ...
... Reactions of β-oxidation 1. Dehydrogenation of fatty acyl CoA to produce a trans double bond between the α and β carbons (or C-2 & C-3). the product is trans-∆2-enoyl-CoA reaction catalyzed by acyl CoA dehydrogenase. the electron acceptor is FAD the reaction is analogous to succinate dehydrogenase 2 ...
Plant Mitochondrial Electron Transfer and Molecular
... the controlled oxidation of metabolites containing reduced carbon to produce carbon dioxide and water as the final products (Taiz and Zeiger, 1991).Severa1 types of reduced carbon compounds, including fatty acids, organic acids, and amino acids, can serve as the primary reducing substrates for plant ...
... the controlled oxidation of metabolites containing reduced carbon to produce carbon dioxide and water as the final products (Taiz and Zeiger, 1991).Severa1 types of reduced carbon compounds, including fatty acids, organic acids, and amino acids, can serve as the primary reducing substrates for plant ...
Plant Mitochondrial Electron Transfer and Molecular
... the controlled oxidation of metabolites containing reduced carbon to produce carbon dioxide and water as the final products (Taiz and Zeiger, 1991).Severa1 types of reduced carbon compounds, including fatty acids, organic acids, and amino acids, can serve as the primary reducing substrates for plant ...
... the controlled oxidation of metabolites containing reduced carbon to produce carbon dioxide and water as the final products (Taiz and Zeiger, 1991).Severa1 types of reduced carbon compounds, including fatty acids, organic acids, and amino acids, can serve as the primary reducing substrates for plant ...
Chapter 3
... Organic Compounds vs Inorganic compounds: • All compounds are classified as either organic or inorganic • Which of the following are organic? (circle them) • Which of the following are inorganic? (underline them) ...
... Organic Compounds vs Inorganic compounds: • All compounds are classified as either organic or inorganic • Which of the following are organic? (circle them) • Which of the following are inorganic? (underline them) ...
Regulation of Primary Metabolism in Response to
... Upon hypoxia, respiratory energy (ATP) production via oxidative phosphorylation by the mitochondrial electron transport chain goes down. To compensate for this, the glycolytic flux increases and Glc is consumed faster in an attempt to produce ATP via the glycolytic pathway, a process known as the Pas ...
... Upon hypoxia, respiratory energy (ATP) production via oxidative phosphorylation by the mitochondrial electron transport chain goes down. To compensate for this, the glycolytic flux increases and Glc is consumed faster in an attempt to produce ATP via the glycolytic pathway, a process known as the Pas ...
Was photosynthetic RuBisCO recruited by
... Geobacillus stearothermophillus; A. vinosum, Allochromatium vinosum; C. limicola, Chlorobium limicola; C. tepidum, Chlorobium tepidum; M. loti, Mesorhizobium loti; S. meliloti, Sinorhizobium meliloti and B. bronchiseptica, Bordetella bronchiseptica. H. marinus, R. capsulatus and A. fulgidus possess ...
... Geobacillus stearothermophillus; A. vinosum, Allochromatium vinosum; C. limicola, Chlorobium limicola; C. tepidum, Chlorobium tepidum; M. loti, Mesorhizobium loti; S. meliloti, Sinorhizobium meliloti and B. bronchiseptica, Bordetella bronchiseptica. H. marinus, R. capsulatus and A. fulgidus possess ...
PowerPoint
... 2. Also consider when AG acidosis and: a) Nl “lactate” levels and no acetone b) Short bowel or other malabsorption syndrome c) Preceded by food ingestion (and symptoms ...
... 2. Also consider when AG acidosis and: a) Nl “lactate” levels and no acetone b) Short bowel or other malabsorption syndrome c) Preceded by food ingestion (and symptoms ...
The Fermentation of Lactic Acid by a Gram
... was dried in a combustion flask and the residue oxidized to C02 (Ormerod, 1956). The C02 produced was assayed for radioactivity as described above. Since the combustion procedure is quantitative, the amount of C02 produced was not measured and specific activities were calculated from the ,moles of a ...
... was dried in a combustion flask and the residue oxidized to C02 (Ormerod, 1956). The C02 produced was assayed for radioactivity as described above. Since the combustion procedure is quantitative, the amount of C02 produced was not measured and specific activities were calculated from the ,moles of a ...
Cobalt Biology Discussion - 1-29-15
... hydratase catalyzes hydration of nitriles to amides, and is a key enzyme involved in the metabolism of toxic compounds (Kobayashi et al., 1992). [4] Glucose isomerase catalyzes the reversible isomeration of D-glucose to D-fructose and is one of the most highly used enzymes in industry (Bhosale et al ...
... hydratase catalyzes hydration of nitriles to amides, and is a key enzyme involved in the metabolism of toxic compounds (Kobayashi et al., 1992). [4] Glucose isomerase catalyzes the reversible isomeration of D-glucose to D-fructose and is one of the most highly used enzymes in industry (Bhosale et al ...
(a) (b)
... which live under aerobic conditions and oxidize their organic fuels to carbon dioxide and water--cellular respiration. First stage, organic fuel molecules are oxidized to yield two-carbon fragments of acetylcoenzyme A (acetyl-CoA). Second stage, the acetyl groups are oxidized to CO2 via TCA pathway; ...
... which live under aerobic conditions and oxidize their organic fuels to carbon dioxide and water--cellular respiration. First stage, organic fuel molecules are oxidized to yield two-carbon fragments of acetylcoenzyme A (acetyl-CoA). Second stage, the acetyl groups are oxidized to CO2 via TCA pathway; ...
Unit 7: Reduction, Oxidation and Electrochemistry
... Examples: Na (s), O2 (g), O3 (g), H2 (g), F2 (g), P4 (s), and Hg (l) all have an oxidation number of 0. 2. Single Atomic Ions have an Oxidation Number Equals to its Charge. Example: K+ has an oxidation number of +1. 3. Oxygen in Binary Compound and Polyatomic Ions has an Oxidation Number of −2. Exam ...
... Examples: Na (s), O2 (g), O3 (g), H2 (g), F2 (g), P4 (s), and Hg (l) all have an oxidation number of 0. 2. Single Atomic Ions have an Oxidation Number Equals to its Charge. Example: K+ has an oxidation number of +1. 3. Oxygen in Binary Compound and Polyatomic Ions has an Oxidation Number of −2. Exam ...
Bio-Organic Mechanism Game – Simplistic biochemical structures
... resonance stabilization in acetal formation or breakdown). Multiple resonance structures are not drawn. Only very occasionally is an intermediate drawn, when confusion arises from too many arrows going in too many different directions. Do not confuse these examples for real mechanisms! They are desi ...
... resonance stabilization in acetal formation or breakdown). Multiple resonance structures are not drawn. Only very occasionally is an intermediate drawn, when confusion arises from too many arrows going in too many different directions. Do not confuse these examples for real mechanisms! They are desi ...
Purification and characterization of the 1-3
... The apparent K m values obtained with substrates and coenzymes were determined at 37°C with potassium carbonate buffer (pH 9.7 for the oxidative reactions and pH 9.1 for the reductive reactions). They were determined from the results of experiments in which a fixed concentration of the substrate or ...
... The apparent K m values obtained with substrates and coenzymes were determined at 37°C with potassium carbonate buffer (pH 9.7 for the oxidative reactions and pH 9.1 for the reductive reactions). They were determined from the results of experiments in which a fixed concentration of the substrate or ...
Direction of Krebs cycle Which way does the citric acid cycle turn
... availability, it is already a textbook definition that NADH oxidized by the respiratory complexes yields NAD+, which will be re-reduced by the dehydrogenases of the cycle. However, as mentioned above, during hypoxia, when the electron transport chain is not operational, NADH may get oxidized by othe ...
... availability, it is already a textbook definition that NADH oxidized by the respiratory complexes yields NAD+, which will be re-reduced by the dehydrogenases of the cycle. However, as mentioned above, during hypoxia, when the electron transport chain is not operational, NADH may get oxidized by othe ...
Lec 16: Nitrogen (ammonia) assimilation
... On the other hand, this reaction occurs in the αKG formation direction for animals because intracellular concentration of NH3 is expected to be low… as NH3 is toxic to animal. (Animals do not utilize NH3 directly as nitrogen source!) ...
... On the other hand, this reaction occurs in the αKG formation direction for animals because intracellular concentration of NH3 is expected to be low… as NH3 is toxic to animal. (Animals do not utilize NH3 directly as nitrogen source!) ...
Short hydrogen bonds in proteins - Molecular Biophysics Unit
... hydroxyl group (OH) orientation (of Ser, Thr and Tyr) being optimized for formation of hydrogen bonds. It is not clear what percentage of these additional SHBs given by hbplus would be retained after energy minimization and how many more would be added to the amber list. However, it is likely that t ...
... hydroxyl group (OH) orientation (of Ser, Thr and Tyr) being optimized for formation of hydrogen bonds. It is not clear what percentage of these additional SHBs given by hbplus would be retained after energy minimization and how many more would be added to the amber list. However, it is likely that t ...
A number of antibiotics produced by different - J
... RNA polymerase. These include the ansamycin group (rifamycins, streptovaricins, tolypomycins and geldanamycin) and streptolydigin, tirandamycin, and thiolutin. The producing organisms of these antibiotics must possess resistance mechanisms to overcome the toxic effects of the antibiotics. Target sit ...
... RNA polymerase. These include the ansamycin group (rifamycins, streptovaricins, tolypomycins and geldanamycin) and streptolydigin, tirandamycin, and thiolutin. The producing organisms of these antibiotics must possess resistance mechanisms to overcome the toxic effects of the antibiotics. Target sit ...
Metabolic Acidosis
... L-Lactic Acidosis Overproduction of L-lactic Acid • Net production of L-lactic acid occurs when the body must regenerate ATP without oxygen • 1 H+ is produced per ATP regenerated from glucose • Because a patient will need to regenerate 72 mmol of ATP per minutes, As much as 72 mmol/min of H+ can be ...
... L-Lactic Acidosis Overproduction of L-lactic Acid • Net production of L-lactic acid occurs when the body must regenerate ATP without oxygen • 1 H+ is produced per ATP regenerated from glucose • Because a patient will need to regenerate 72 mmol of ATP per minutes, As much as 72 mmol/min of H+ can be ...
Scott et al. 2006
... it was possible to identify key aspects of intermediary carbon metabolism in the microbial world. In fact, microorganisms examined in this study could be placed within one of three metabolic groups: (1) heterotrophs that grow by oxidizing compounds containing three or more carbon-to-carbon bonds (fe ...
... it was possible to identify key aspects of intermediary carbon metabolism in the microbial world. In fact, microorganisms examined in this study could be placed within one of three metabolic groups: (1) heterotrophs that grow by oxidizing compounds containing three or more carbon-to-carbon bonds (fe ...
Bio 226: Cell and Molecular Biology
... Make fatty acids in plastids with a prokaryotic FAS • 12 proteins, instead of one multifunctional protein • Assemble some lipids in CP, others in ER • Acetyl-CoA carboxylase is also prokaryotic = 4 subunits, except in grasses (profoxydim & other grass herbicides inhibit ACCase) ...
... Make fatty acids in plastids with a prokaryotic FAS • 12 proteins, instead of one multifunctional protein • Assemble some lipids in CP, others in ER • Acetyl-CoA carboxylase is also prokaryotic = 4 subunits, except in grasses (profoxydim & other grass herbicides inhibit ACCase) ...
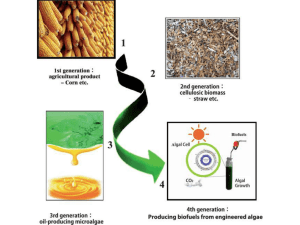
Microbial metabolism
Microbial metabolism is the means by which a microbe obtains the energy and nutrients (e.g. carbon) it needs to live and reproduce. Microbes use many different types of metabolic strategies and species can often be differentiated from each other based on metabolic characteristics. The specific metabolic properties of a microbe are the major factors in determining that microbe’s ecological niche, and often allow for that microbe to be useful in industrial processes or responsible for biogeochemical cycles.== Types of microbial metabolism ==All microbial metabolisms can be arranged according to three principles:1. How the organism obtains carbon for synthesising cell mass: autotrophic – carbon is obtained from carbon dioxide (CO2) heterotrophic – carbon is obtained from organic compounds mixotrophic – carbon is obtained from both organic compounds and by fixing carbon dioxide2. How the organism obtains reducing equivalents used either in energy conservation or in biosynthetic reactions: lithotrophic – reducing equivalents are obtained from inorganic compounds organotrophic – reducing equivalents are obtained from organic compounds3. How the organism obtains energy for living and growing: chemotrophic – energy is obtained from external chemical compounds phototrophic – energy is obtained from lightIn practice, these terms are almost freely combined. Typical examples are as follows: chemolithoautotrophs obtain energy from the oxidation of inorganic compounds and carbon from the fixation of carbon dioxide. Examples: Nitrifying bacteria, Sulfur-oxidizing bacteria, Iron-oxidizing bacteria, Knallgas-bacteria photolithoautotrophs obtain energy from light and carbon from the fixation of carbon dioxide, using reducing equivalents from inorganic compounds. Examples: Cyanobacteria (water (H2O) as reducing equivalent donor), Chlorobiaceae, Chromatiaceae (hydrogen sulfide (H2S) as reducing equivalent donor), Chloroflexus (hydrogen (H2) as reducing equivalent donor) chemolithoheterotrophs obtain energy from the oxidation of inorganic compounds, but cannot fix carbon dioxide (CO2). Examples: some Thiobacilus, some Beggiatoa, some Nitrobacter spp., Wolinella (with H2 as reducing equivalent donor), some Knallgas-bacteria, some sulfate-reducing bacteria chemoorganoheterotrophs obtain energy, carbon, and reducing equivalents for biosynthetic reactions from organic compounds. Examples: most bacteria, e. g. Escherichia coli, Bacillus spp., Actinobacteria photoorganoheterotrophs obtain energy from light, carbon and reducing equivalents for biosynthetic reactions from organic compounds. Some species are strictly heterotrophic, many others can also fix carbon dioxide and are mixotrophic. Examples: Rhodobacter, Rhodopseudomonas, Rhodospirillum, Rhodomicrobium, Rhodocyclus, Heliobacterium, Chloroflexus (alternatively to photolithoautotrophy with hydrogen)