* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Bio-Organic Mechanism Game – Simplistic biochemical structures
Metabolic network modelling wikipedia , lookup
Radical (chemistry) wikipedia , lookup
Nicotinamide adenine dinucleotide wikipedia , lookup
Oxidative phosphorylation wikipedia , lookup
Microbial metabolism wikipedia , lookup
Photosynthesis wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Citric acid cycle wikipedia , lookup
Multi-state modeling of biomolecules wikipedia , lookup
Biosynthesis wikipedia , lookup
Metalloprotein wikipedia , lookup
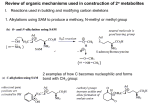
1 Bio-Organic Mechanism Game – Simplistic biochemical structures and simplistic organic reaction mechanisms are used to explain common biochemical transformations. Simplified biochemical molecules are presented first. Many biomolecules have a somewhat complex structure that makes it difficult to write out step by step mechanisms. However, if we simplify those structures to the essential parts necessary to explain the mechanistic chemistry of each step, it becomes much easier to consider each step through an important cycle. I have proposed possible simplified structures that are used in the later examples of biochem cycles and problems. The usual strategy in biochem cycles is to just write names, or perhaps, names and a structure. Occasionally a few mechanistic steps are suggested, but almost never is a detailed sequence of mechanistic steps provided. Since it is hard to find such detailed mechanistic steps anywhere (sometimes they are not known) our proposed steps are, of necessity, somewhat speculative. In this book we are not looking for perfection, which is not possible, but for sound organic logic that is consistent with the biochemical examples presented below. There is great satisfaction in blending organic knowledge with real life reactions that help explain how life works. In working through some of the problems, you may develop an alternative mechanism that is just as good, or even better than the one I have proposed. If you do, I hope you will share it with me and if an improved version of this book ever gets written I can include it the next edition (and give you credit). It is almost certain that I have made some errors and I would appreciate it if you would let me know about them. Biomolecules and our simplified representation. 1. ATP – adenosine triphosphate – phosphorylation, energy source NH2 N O O P O O ATP O = O P O O O P O O simplified structure N O P O CH2 O H H OH H OH H actual structure N O N 2. NAD+ and NADP+ - nicotinamide adenine dinucleotide (hydride acceptor) NH2 CONH2 N = N R N H 2C P O P O CH2 OH HO O O simplified structure O actual structure y:\files\classes\Organic Hunger Games\Bio-Org Game newer.doc N O O O N O N H H OH H OH NADP+ has a H phosphate here. 2 3. NADH and NADHP - nicotinamide adenine dinucleotide (hydride donor) H H H NH2 CONH2 H N O = N H2C N OH HO R O O P N O O O P O CH2 O N O H H OH H OH H simplified structure N NADPH has a phosphate here. 4. Vitamin B-6 – pyridoxal phosphate (amino acid metabolism, transamination with -ketoacids, decarboxylation, removal of some amino acid side chains, epimerizations) 1o amine version of Vit B-6 NH2 aldehyde version of Vit B-6 NH2 H H O -2 O3PO O H simplified structure O H interconvert via imine, tautomers, hydrolysis O -2 O3PO = N H = N N H H H actual structure simplified structure actual structure N 5. TPP – Thiamine diphosphate (decarboxylation and enamine chemistry with proton or carbohydrates) N N B N simplified structure N = H S N R R NH2 ylid form of TPP S O P O y:\files\classes\Organic Hunger Games\Bio-Org Game newer.doc H O O S O P O O CH2 actual structure 3 6. Coenzyme A (acyl transfer) All of this is "Co-A" O O O S O N H H P N H O O P O N O O N OH Thiol esters form here. H NH2 O O N N HO OH CoA S CoA This is an acetyl group simplified structure of CoA S actual structure simplified structure of acetyl Co-A 7. FAD / FADH2 – Flavin adenine dinucleotide (oxidation – reduction) – used to deliver hydride to C=C or take hydride from CH-CH (fatty acid metabolism, etc.) O O P O O P O N NH2 O O O N HO OH N actual structure N HO OH OH N O N FAD - flavin dinucleotide (a hydride acceptor) NH N H O N N N N FAD H FADH2 simplified structures for FAD and FADH2 y:\files\classes\Organic Hunger Games\Bio-Org Game newer.doc 4 H H B N C B H C C H H N C N FAD flavin dinucleotide (a hydride acceptor) H C N N H C N FADH2 B H flavin dinucleotide (a hydride donor) Hydride transfer reduces FAD to FADH2 which can be oxidized to FAD. B 8. THF – tetrahdrofolate (transfer of one carbon units) –recycles cysteine to methionine and other 1C metabolic functions, many variations Tetrahydrofolate (THF) one carbon transfers as "CH3", "CH2". R H2N R N V22-p762 N H N actual structure N H = 5 N H HN 10 Ar simplified structure N H O One glutamate is shown, but several can be attached. HN One carbon groups can bond here in various ways (see below structures. y:\files\classes\Organic Hunger Games\Bio-Org Game newer.doc H N O CO2 CO2 5 R different forms R R R N N N N NAD+ 5 NADH N HN N5-methyl THF 5 -H2O N O N H2C Ar 5 N H 5 NADPH N CH3 +H2O NADP+ 10 N HC Ar N ,N -methylene THF glycine or serine THF -H2O 10 10 Ar N10-formyl THF Ar H N5,N10-methenyl THF 10 N HCO2 ATP +H2O R +NH3 R N -NH3 N histidine THF N THF 5 5 N HN H O HN NH 10 H Ar 10 Ar N -formimino =THF 5 N5-formyl THF 9. SAM = S-adenosylmethionine (methyl transfer agent), The methyl group (CH3) attached to the methionine sulfur atom in SAM is chemically reactive. This allows donation of this group to an acceptor substrate in transmethylation reactions. More than 40 metabolic reactions involve the transfer of a methyl group from SAM to various substrates, such as nucleic acids, proteins, lipids and secondary metabolites. SAM can be made from methionine and N5-methyl THF (just above). leaving group was triphosphate SAM = S-adenosylmethionine Rmet N methionine NH2 Rad NH2 O O S CH3 N S N = OH simplified structure actual structure Rmet = methionine Rad = adenonine O NH2 O O P O O HO OH adenosine SAM = S-adenosylmethionine O O P O N CH3 N O NH2 O P O N O S N N OH methionine CH3 y:\files\classes\Organic Hunger Games\Bio-Org Game newer.doc HO OH ATP 6 10. Cytochrom P-450 enzymes are oxidizing agents in the body. They can convert inert alkane sp3 C-H bonds into C-OH bonds and they can make epoxide groups at alkenes and aromatic pi bonds. Oxidations in the body often use cytochrom P-450 enzymes. N N N +3 Fe N N simplified structure N simplified structure Fe N N +3 N Fe N N N S S Enz Enz HO2C CO2H heme, protoporphyrin IX, found in cytochrom P-450 oxidative enzymes This is the structure that we will use. O +4 Fe +3 Fe simplified structure 11. Halogenase Enzymes (related to cytochrom P-450 enzymes, can have imidazole ligands from histadine amino acids Halogenations in the body often iron halogen bonds. His N O N N O N This is the structure that we will use. Cl O O +4 Fe +3 Fe Fe His H Cl simplified structure +3 N Fe N +4 Cl N N N His Biochemical Reaction Mechanism Examples Mechanism arrows used in the “Bio-Org Game” are meant to suggest how the electrons move over a single transformation, and are not necessarily meant to imply that all of the electrons and atoms transfer in one huge “domino” cascade. Organic mechanisms are often multistep transformations, but it’s harder to pin down biochemical transformations. The symbolism used in these examples represents a concise way to show electron movement involving making and breaking bonds. Lone pairs are rarely drawn (or used). They are included on the generic base (B:) used to show proton transfers. A generic acid (H-B+) is used to provide a proton. Very occasionally a pair of electrons is used when it provides some special effect (enamine reaction, y:\files\classes\Organic Hunger Games\Bio-Org Game newer.doc 7 resonance stabilization in acetal formation or breakdown). Multiple resonance structures are not drawn. Only very occasionally is an intermediate drawn, when confusion arises from too many arrows going in too many different directions. Do not confuse these examples for real mechanisms! They are designed to show the essential how changes might occur in complex biochemical reactions. Also, at physiological pH (7) a few organic groups are ionized (RCO2H is anionic as RCO2--, and RNH2 is cationic as RNH3+). They are drawn in their neutral forms in this game. The initial examples of biochemical transformations can serve as foundational reactions in endless biochemical sequences or cycles. Knowing how these reactions work can provide insight into many biochemical aspects of anabolism and catabolism and can help improve your organic “mechanistic” logic. First, bare-bones examples are provided to show the essence of each type of reaction. The problems that follow use several “typical” types of biochemical transformations in made-up sequences and many real biochemical cycles in which to practice. With such practice using these simple model reactions you can learn to recognize where (when) and how similar transformations might be occurring in real biochemical reactions that are presented without any mechanistic detail in a book or article. Nature uses simple strategies applied to limited classes of molecules (carbohydrates, lipids, fats, steroids, amino acids, nucleic acids, neurotransmitters, alkaloids, terpenoids and more) having enormous variation of patterns. It’s amazing what you can speculate upon using these few reactions. An example of mechanistic simplification. An “organic” arrow pushing mechanism, showing keto enol tautomerization in acid, is shown without simplification, having all of the normal mechanistic details (lone pairs, formal charge, resonance, etc.). We won’t do it this way in the Bio-Org game. H B O H C C O O O C H H H C H C B C H C C resonance A complete organic mechanism shows lone pairs, each individual step and resonance structures. The same mechanism in the Bio-Organic Mechanism Game is shown in the first “biochem” example, using the simplified mechanistic conventions of this game. 1. Keto / enol tautomerization (two proton transfers and a shift of pi electrons). H B B H B = general base, possibly on enzyme O O B C H C keto tautomer y:\files\classes\Organic Hunger Games\Bio-Org Game newer.doc H C C enol tautomer B H B = general acid, possibly on enzyme 8 H B H H O B B O C C C H B C enol tautomer keto tautomer Quite often in biochemistry the acid and base functions are a cooperative action in the active site of an enzyme, much in the manner used in this Game. This avoids the necessity of very strong acid or very strong base, often used by chemists in their reactions. Such conditions are not tolerated by living organisms. We arbitrarily use neutral base, B: and cationic acid H-B+. 2. Carbonyl hydration – a regioselective addition reaction of H2O to a carbonyl group. This also requires some proton tranfers. A carbonyl hydrate can be dehydrated via an elimination reaction which also requires some proton transfers. These steps are very similar to hemiacetal/hemiketal reactions (Example 6), but use H-O-H instead of R-O-H. The carbonyl hydrate can be used to oxidize an aldehyde (example 8) or allow a reverse aldol reaction (example 3). Forward Direction B O B B H H O H H hydration C H O H O C (addition reaction) carbonyl hydrate aldehyde or ketone Reverse Direction B B H H O O C H B H O dehydration (elimination reaction) carbonyl hydrate B H O H C aldehyde or ketone 3. Aldol reactions make a new carbon-carbon bond, forming a -hydroxycarbonyl compound. A carbonyl C position becomes the nucleophile (as enol or enolate) and reacts with a separate electrophilic carbonyl carbon. Reverse aldol reactions cleave the C-C bond, leaving the electrons on a C position and forming a C=O at the C-OH position. The aldol product can proceed on an additional step as shown in Example 5 (reverse Michael ,-unsaturated carbonyl compounds) y:\files\classes\Organic Hunger Games\Bio-Org Game newer.doc B 9 Forward Aldol B B B H H C C C This product also looks like a Michael reaction. See Example 5. -hydroxycarbonyl carbonyl nucleophile at C position carbonyl electrophile B C C C H O O aldol (addition reaction) O O H Reverse Aldol B H H O O C C B reverse aldol (elimination reaction) B B H O O H C C C C -hydroxycarbonyl 4. Claisen reactions make a new carbon-carbon bond, forming -ketocarbonyl compounds. A carbonyl C position becomes the nucleophile (as enol or enolate) and reacts with a separate electrophilic carbonyl carbon (similar to Example 3, except carbonyl substitution occurs instead of carbonyl addition). Ester groups are common in organic chemistry and thiol ester groups (acetyl Co-A) are common in biochemistry. Reverse Claisen reactions cleave the C-C bond leaving the electrons on the C position and forming a carboxyl at the C=O position. The tetrahedral intermediate is omitted in the Bio-Organic Game. Forward Claisen H B B B O H C Claisen (acyl substitution) C C OR OR C R C H H B reverse Claisen (acyl substitution) C C R O alcohol or thiol -ketocarbonyl ester or thiol ester B O O O OR O Reverse Claisen -ketocarbonyl OR B y:\files\classes\Organic Hunger Games\Bio-Org Game newer.doc B O alcohol or thiol carbonyl nucleophile H C C H carbonyl electrophile with a leaving group, (often and ester or thiol ester or a mixed anhydride) O O O H C C OR carbonyl electrophile with a leaving group, (often and ester or thiol ester) C OR ester or thiol ester H B 10 5. Reverse Michael reaction (elimination = dehydration) eliminates H2O between the C-C bonds (E1cB mechanism). Dehydration carries the aldol (Example 3) one step farther along, forming ,-unsaturated carbonyl compounds. Michael reaction (addition / hydration) is the reverse reaction and adds the elements of water across the C=C, a resonance extension of the C=O. We have taken liberties with the name “Michael”. It is probably better to describe these reactions as conjugate addition and reverse conjugate addition. A close variation of this reaction eliminates alcohols instead of water. Other possibilities also exist. Reverse Michael Reaction B B H H O C O O C C C C C (elimination) H -hydroxycarbonyl -unsaturated carbonyl (Michael acceptor) B Reverse Michael Reaction H B H H reverse Michael reaction O H B O O O Michael reaction C C H H C C H C C (addition) -unsaturated carbonyl (Michael acceptor) O H -hydroxycarbonyl B B H B This product looks like an aldol product. See Example 3. It is now able to do a reverse aldol, or be oxidized to a 1,3-dicarbonyl, maybe followed by decarboxylation, etc. 6. Hemiacetal (or hemiketal) formation is an addition reaction to a carbonyl by alcohol, similar to carbonyl hydration and dehydration, Example 2. The reverse reaction reforms the carbonyl group and alcohol in an elimination reaction., A second alcohol can react with the hemiacetal/ketal and undergo an SN1 reaction with the OH to form an acetal or ketal (Example 7, just below). The example shown here is an intramolecular reaction and typically forms rings of 5 or 6 atoms. Forward Direction B H H O O C C alcohol aldehyde or ketone H B y:\files\classes\Organic Hunger Games\Bio-Org Game newer.doc B addition reaction H O O hemiacetal / hemiketal B 11 Reverse Direction B H H B B H B H O O elimination reaction O O C C alcohol hemiacetal / hemiketal aldehyde or ketone 7. Acetal (or ketal) formation from a hemiacetal (or hemiketal). The “OH” becomes a water molecule leaving group that is replaced by an “OR” in an SN1 reaction, producing an ether linkage. In the reverse reaction (acetal or ketal forming a hemiacetal or hemiketal) an alcohol leaving group is replaced by a water molecule in an SN1 reaction. These are reversible reactions that require acid catalysis. Because arrows are used in both directions on the same bonds, we show the intermediate in this example. These reactions often occur when one sugar molecule “OH” connects to another sugar molecule at its hemiacetal site (such as galactose + glucose = lactose). Such linkages can go on for hundreds of sugar molecules (glycogen in animals and cellulose in plants). Forward Direction B B H H H R H O O H R O O O O O eliminate H2O hemiacetal / hemiketal add ROH acetal / ketal intermediate Reverse Direction H B B B H R O O O add H2O eliminate ROH acetal / ketal H O O O H H intermediate hemiacetal / hemiketal 8. a. Oxidation of CH(OH) to C=O (1o ROH aldehyde, 2o ROH ketone, hydrated aldehyde carboxylic acid) with an equivalent of NAD+. NAD+ accepts a hydride via conjugate addition, quenching the positive charge on the nitrogen and forms NADH. A base removes a proton from the adjacent oxygen atom allowing an elimination reaction to produce the C=O (or in Example 9, a C=N). y:\files\classes\Organic Hunger Games\Bio-Org Game newer.doc 12 H B H B H O H oxidation of an alcohol C H N C O N elimination reaction R R + NAD equivalent 1o or 2o alcohol H aldehyde or ketone NADH equivalent b. Reduction of C=O to CH(OH) with an NADH equivalent is the opposite of the above reaction. NADH is a hydride donor that becomes aromatic (forms NAD+) with the transfer of the nucleophilic hydride to the electrophilic C=O. A nearby acid protonates the oxygen completing the addition reaction. B H H H H B H O reduction of a carbonyl C N addition reaction O H C N R NADH equivalent aldehyde or ketone 1o or 2o alcohol R NAD+ equivalent 9. a. Oxidation of an amine, CH(NHR), to imine (C=N-R) with an NAD+ equivalent that is reduced to NADH, followed by hydrolysis to a C=O. This is the opposite of 9b, below. The first step is similar to reaction 8a above with an alcohol. Overall, this is a transformation of an amine into a carbonyl group and a primary amine. Oxidation of an amine B R N H H H H B elimination reaction C R C H N amine R NAD+ equivalent y:\files\classes\Organic Hunger Games\Bio-Org Game newer.doc H N N oxidation of an amine imine R NADH equivalent 13 Hydrolysis of an imine B B H H B H H R O N R H R O C elimination reaction addition reaction imine B H C C H N O N B H B H aminal aldehyde or ketone b. Formation of an imine, C=N-R, from a C=O, followed by reduction to CH(NHR) (an amine) with an NADH equivalent that is oxidized to NAD+. This is the opposite of 9a, above. The second step is similar to reaction 8b above with an alcohol. Overall, this is a transformation of a carbonyl group into an amine. Formation of an imine B B H H R N H B O R H H B R H N C aldehyde or ketone B B O N addition reaction C H H elimination reaction aminal O C H imine Reduction of an imine B H H H B R H H reduction of an imine N N R C N imine R NADH equivalent H addition reaction C N amine NAD+ equivalent R 10. Decarboxylation of a -ketocarboxylic acid, forming an enol, which tautomerizes to a keto group. H O B B H O O C decarboxylation O C O -ketocarboxylic acid enol tautomer y:\files\classes\Organic Hunger Games\Bio-Org Game newer.doc B H C C C H C B H O O carbon dioxide tautomers C H C keto tautomer B B 14 11. Decarboxylation of an -ketocarboxylic acid with TPP (thiamine diphosphate). Also includes both “TPP ylid” and “TPP enamine” chemistry. The enamine can be protonated to form an aldehyde or it can react with another carbonyl compound to make a new carbon-carbon bond (a larger carbohydrate in this game) The TPP ylid is also regenerated, which can react again or protonate to make TPP. See another reaction of -ketocarboxylic acids with Vit B-6 in Example 13. TPP reaction with an a-ketoacid, decarboxylation and formation of enamine. R OH R B N O H B R O N C H S S TPP pKa 18 O R -ketoacid TPP ylid B H C O C N C TPP ylid addition to a carbonyl S OH R decarboxylation (-CO2) R OH N C S R TPP enamine Reaction of enamine nucleophile with a carbonyl electrophile followed by an E2-like reaction to from a new (larger) carbohydrate. B R R R H H B O O OH N N OH N C C S H R TPP enamine reaction with C=O C R TPP enamine addition to a carbonyl S R C H R elimination reaction forms a carbonyl group, similar to a reverse aldol S TPP ylid O OH C R CH CH R OH a new (larger) carbohydrate y:\files\classes\Organic Hunger Games\Bio-Org Game newer.doc 15 Reaction of enamine nucleophile with an acid to from a new (smaller) carbohydrate. B R R O N OH N H C B O N C S S R TPP enamine reaction with a proton R H R acid/base protonation of TPP enamine C H H R S elimination reaction forms TPP ylid a carbonyl group, similar to a reverse aldol a new (smaller) carbohydrate 12. a. Phosphorylation of an OH with an ATP equivalent (making an inorganic phosphate ester). O O O O O P O O O P O P O P O O C O O O O +2 Mg H B P O ADP ATP acyl-like substitution reaction O Mg+2 ADP = leaving group O Complexing with Mg+2 can make one phosphorous atom more electrophilic and the other one a better leaving group. The Mg+2 is not required to show this reaction. Mg+2 is not used in the other reactions below, but it could be. C O P O O H B inorganic phosphate ester b. Dephosphorylation of an inorganic phosphate ester to an alcohol and phosphate. B H O C O P O H B inorganic ester hydrolysis O O C O B O O P H acyl-like substitution reaction H H H O O B c. An elimination reaction of a phosphate leaving group to make an alkene (pi bond). Could actually be E1 or E2. O C O P O O E1 or E2 (anti) mechanisms are possible C C C B H O H O H y:\files\classes\Organic Hunger Games\Bio-Org Game newer.doc B H P O O 16 d. acyl phosphate (inorganic anhydride) and formation of a thiol ester (like acetyl Co-A) ADP leaving group O O O P O P O phosphate leaving group ADP O O O O O Mg+2 O P O P organic-inorganic anhydride ATP O Mg+2 O C acyl-like substitution reaction H H3C O O O O B O C O P H3C O O O Mg+2 acyl-like substitution reaction H B Co-A Co-A S very reactive thiol ester (acetyl Co-A) S H B 13. Vit B-6 reactions – 1. imine formation with -keto acid and the amino version of Vit B-6 (similar to Example 9b), 2. tautomerization (Example 1) and 3. imine hydrolysis to amino acid and the aldehyde version of Vit B-6 (similar to Example 9a). B H OH B H H O OH OH O N N H -keto acid R B N H vitamin B-6 (1o amine version) N O R O imine synthesis N H Similar to Example 9b. y:\files\classes\Organic Hunger Games\Bio-Org Game newer.doc H O R H B dehydration (-H2O) N H O H H 17 B OH OH H H B H N N O O H Similar to Example 1. R R keto / enol tautomerization, makes imine on the other side N N H H B OH H OH H H O BH H B H H B H2N OH O H H O N O N O O R H N N H H y:\files\classes\Organic Hunger Games\Bio-Org Game newer.doc hydrolysis of imine R -amino acid R addition of water Similar to Example 9a. H N H vitamin B-6 (aldehyde version) 18 Three additional vit B6 reactions from aromatic imine 1. Decarboxylation B H O B C O H O H N N B H H BH H H N O R H O R H R decarboxylation N N H H N H B O H H H H H N O N B H R R N H N H y:\files\classes\Organic Hunger Games\Bio-Org Game newer.doc 19 2. Elimination of side R group, like serine or threonine. B serine aa H2C B H O H O H N H O B N H BH N CO2H CO2H CO2H decarboxylation N N H vitamin B-6 (imine version) N H H H B H also threonine aa O O O N H H H H N O N H H H CO2H B CO2H N CO2H glycine aa N H N glycine aa CO2H H H vitamin B-6 (aldehyde version) N H 3. Epimerize a proton at the C position of an amino acid. S amino acid N H B proton adds on the opposite face R N CO2H N H vitamin B-6 (imine version) y:\files\classes\Organic Hunger Games\Bio-Org Game newer.doc H B R amino acid N R R H CO2H CO2H N N H H vitamin B-6 (imine version) 20 14. FAD / FADH2 reduction of C=C to CH-CH or the reverse reaction oxidation of CH-CH to a C=C, FAD can be recharged with NADH. Simplified mechanism of action for reduction of C=C by FADH2 FADH2 from NADH. FAD and mechanism for reforming O O H H CoA R CoA S R H H N S H H B B N H N FADH2 B B N FAD H H H H N N N N NAD+ N R B H N NADH FADH2 H R FAD H H B N N FADH2 - flavin dinucleotide (a hydride donor) FAD - flavin dinucleotide (a hydride acceptor) used to reoxidize fats for energy. N N H H O H H C R H H C C S C O Enz C R FADH2 B A saturated fatty acid chain. y:\files\classes\Organic Hunger Games\Bio-Org Game newer.doc C Enz S H An ,-unsaturated fatty acid chain. 21 3 3 15. Cytochrom P-450 oxidation of sp C-H bonds to make sp C-OH groups alcohols sp3 C-H bonds H O H C H O O C C Fe +3 Fe +4 9 Fe +4 8 The carbon free radical abstracts hydroxyl (OH) from iron, making an C-OH bond where a C-H bond had been. The iron is reduced back at Fe+3 to begin the process all over again. The free radical-like oxygen atom abstracts a hydrogen atom from a C-H bond in the enzyme cavity, forming an O-H bond and a carbon free radical. 16. Cytochrom P-450 oxidation of C=C pi bonds (alkenes and aromatics) to make epoxides, which can be opened to diols. R R R C R C C R C C R O O R R R R R C O R epoxides Fe +3 Fe +4 Fe +4 The free radical-like oxygen atom adds to a C=C bond (alkene or aromatic) in the enzyme cavity, forming a O-C bond and a carbon free radical. The carbon free radical abstracts the oxygen atom from the iron, making an epoxide ring. The iron is reduced back at Fe+3 to begin the process all over again. Reactive epoxides can be opened up to diols (more water soluble). 17. Cytochrom P-450 oxidation of sulfur and nitrogen lone pairs. S S R R O O sulfur substrate (1e-) sulfur substrate Fe +4 Fe +4 N S R R R/H N R R Fe +4 Fe +3 sulfoxides, further oxidation is possible, all the way to sulfate, SO4-2 R/H O O R R nitrogen substrate (1e-) y:\files\classes\Organic Hunger Games\Bio-Org Game newer.doc O R nitrogen substrate Fe +4 O R Fe +3 N R R/H R N-oxides, further oxidation is possible, all the way to nitrate, NO3-1 22 18. Halogenation of sp3 C-H bonds to make C-X groups (X = Cl, Br, I) using halogenase enzymes. sp3 C-H bonds H C O Cl H C Cl Fe +5 The free radical-like oxygen atom abstracts a hydrogen atom from a C-H bond in the enzyme cavity, forming an O-H bond and a carbon free radical. H O C O Cl Fe +5 Fe +4 The carbon free radical abstracts a halogen (Cl or Br) forming an unusal halogenated bioorganic molecule. 19. Halogenations of aromatic rings using X-OH to make sp2 C-X bonds (like thyroxine). Iodide is stored in the thyroid gland. Iodoperoxidase enzyme makes it electrophilic (instead of nucleophilic iodide). It could react as hypoiodous acid, or the oxygen could be made into an even better leaving group if was a phosphate (using ATP). H B H O H O I O O O H O H P O ATP O O I H I O B P O O possibly an even better leaving group a good leaving group on iodine Speculative mechanism for iodinating tyrosine and formation of thyroxine from two tyrosines. O I B H I CO2H CO2H H H NH2 H O NH2 O tyrosine I CO2H H NH2 O I repeat H NH2 O I diiodotyrosine - makes thryoxine y:\files\classes\Organic Hunger Games\Bio-Org Game newer.doc CO2H 23 20. S-adenosyl methionine (SAM-e) to methylate biomolecules. NH2 B 6 H Rmet S H3C N 1 5 NH2 NH2 H3C N H N 2 CH3 4 Rad N N O 3 N O O H H H 5-methylcytosine cytosine 21. Anti-oxidation reactions using vit E (fat soluble), vit C (water soluble), (also possible are glutathione, resveratrol and other bio-antioxidants). possible dangerous free radical reduced by vit E reduced radical neutralized by body's buffer system R R R H H H B R O H R O free radical protection by vitamin E (possibly in cell membrane) R O H R R R O O O R R H O H vitamin C reduces vitamin E back to normal and ultimately washes out of the body H B O R O resonance and inductive effects stabilize radical so it does not do damage vitamin E located in cell membranes quenches radicals O H O H O H O O H O O H O O O R R O R O B O R H O B protects a second time O O oxidized vitamin C form washes out of the body O O R y:\files\classes\Organic Hunger Games\Bio-Org Game newer.doc R H O R vitamin E is recharged and still in cell membrane 24 other possible anti-oxidants OH glutathione SH NH2 O H O N N OH OH HO resveratrol O glutaric acid ( linkage) H OH O cysteine glycine 3 types of problems are possible 1. Fill in the missing mechanistic details. 2. State what transformation occurred (and provide the missing mechanistic details). 3. Given the term, draw the step (and provide the missing mechanistic details). Summary of Biochemical Topics having examples provided above: 1. Keto/enol tautomerization (proton transfer, resonance, proton transfer). 2. Carbonyl hydration (addition reaction of H2O to a C=O) / carbonyl hydrate dehydration (elimination reaction forms a C=O and H2O). 3. Aldol (makes a -hydroxy carbonyl compound). Reverse aldol, (makes 2 C=O from a -hydroxy carbonyl compound). 4. Claisen (makes a -keto ester). Reverse Claisen (makes two esters). Often occurs using thiol esters in biochemistry (such as acetyl Co-A). 5. Reverse Michael reaction (elimination / dehydration) of -hydroxy carbonyl compounds, an elimination reaction forms ,-unsaturated carbonyl compounds and carries an aldol reaction one step farther. Michael reaction (addition / hydration) adds a nucleophile at the beta carbon of an ,-unsaturated carbonyl compound (usually OH in this game) and adds a proton at the alpha carbon. 6. Hemiacetal (or hemiketal) formation (an addition reaction) of an alcohol to a C=O, forms an ether and an alcohol group on the same carbon. The reverse reaction reforms the carbonyl and alcohol from an elimination reaction. Typical ring sizes in the intramolecular reaction are 5-6 atoms. These transformations are very similar to carbonyl hydration / dehydration, presented in example 2 above. 7. Acetal (or Ketal) formation from a hemiacetal (or hemiketal) makes a water molecule leaving group that is replaced by an alcohol in an SN1 reaction, producing a second ether linkage. In the reverse reaction (acetal or ketal forming a hemiacetal or hemiketal) an alcohol leaving group is replaced by a water molecule in an SN1 reaction. Both are reversible reactions. Because arrows are used in both directions on the same bonds, we show the intermediate in this reaction. 8. a. Oxidation of CH(OH) to C=O with an NAD+ equivalent, which is reduced to NADH (opposite of 8b, below). y:\files\classes\Organic Hunger Games\Bio-Org Game newer.doc 25 + b. Reduction of C=O to CH(OH) with an NADH equivalent, which is oxidized to NAD (opposite of 8a, above). 9. a. Oxidation of an amine, CH(NHR), to C=N-R (an imine) with an NAD+ equivalent which forms NADH, followed by imine hydrolysis to a C=O (opposite of 9b, below). b. Formation of an imine, C=N-R, from a C=O, followed by reduction to CH(NHR) (an amine) with an NADH equivalent which forms NAD+ (opposite of 9a, above). 10. Decarboxylation of a -ketocarboxylic acid, liberates CO2 and forms an enol which tautomerizes to a keto group. 11. Decarboxylation of an -ketocarboxylic acid with TPP (thiamine pyrophosphate = diphosphate). The “TPP ylid” adds to an -keto group, liberates CO2 and becomes a “TPP enamine”, which can protonate or react with a C=O of another carbohydrate. 12. a. Phosphorylation of an OH with an ATP equivalent (making a phosphate ester) – common in enzyme signaling b. Dephosphorylation of a phosphate ester to an alcohol and phosphate – common in enzyme signaling c. Making an alcohol OH into a phosphate ester makes it a better leaving group. An elimination (E1 or E2) or substitution (SN2) reaction with a phosphate leaving group is possible. d. Formation of acyl phosphates (mixed anhydrides) also allows for exothermic carbonyl substitution reactions (can make thiol esters). 13. Vit B-6 reactions – Many reactions are possible. The only example shown is 1. imine formation with an -keto acid and the primary amine version of Vit B-6, 2. tautomerization to a different imine, and 3. imine hydrolysis to an amino acid and the aldehyde version of Vit B-6. The imine complex also allows for the loss of various groups on amino acids (the acid part, CO2H, an alpha C-H proton, and certain amino acid side groups, -CH2OH in serine, and -CHCH3OH in threonine). Imines are also seen in Example 9 and -keto acids in Example 11. 14. FAD / FADH2 reduction of C=C to CH-CH or the reverse reaction oxidation of CH-CH to a C=C, FAD can be recharged with NADH. 15. Cytochrom P-450 oxidation of sp3 C-H bonds to make sp3 C-OH groups. 16. Cytochrom P-450 oxidation of C=C pi bonds (alkenes and aromatics) to make epoxides, which can be opened to diols. 17. Cytochrom P-450 oxidation of sulfur and nitrogen lone pairs. 18. Halogenation of sp3 C-H bonds to make C-X groups (X = Cl, Br, I) using Fe halogenase enzymes. 19. Halogenations of aromatic rings using X-OH to make sp2 C-X bonds (X = Cl, Br, I) (thyroxine). 20. S-adenosyl methionine (SAM-e) to methylate biomolecules. 21. Anti-oxidation reactions using vit C, vit E, resveratrol, glutathione, and other bio-antioxidants. y:\files\classes\Organic Hunger Games\Bio-Org Game newer.doc 26 Many of the steps of biochemical cycles can be explained with the above reactions. Problem – Use B-H+ / B: and any necessary cofactors to accomplish the following transformations using simplistic mechanisms (you do not need to show lone pairs of electrons and you can combine multiple steps using several arrows). 1. H H O H O O H O O H O O H reverse aldol reverse those steps, do a forward aldol (acyl substitution reaction) (acyl substitution reaction) hemiacetal formation 6 atom ring reverse that reaction back go a carbonyl and an alcohol 2. H H O H O O H O O H O (addition reaction) O (elimination reaction) H 3. H H O H O H O O reverse Michael (dehydration) O O H O H (elimination reaction) forward Michael (hydration) (addition reaction) 4. H H O H O H O O keto/enol tautomerization to form an aldehyde (twice) O O H O carbonyl (hydration) (addition reaction) H 5. H H O H O H O O keto/enol tautomerization to form a new ketone (twice) O O H O H y:\files\classes\Organic Hunger Games\Bio-Org Game newer.doc hemiacetal formation 5 atom ring (addition reaction) 27 6. OH OH O keto/enol tautomerization to form a beta keto acid OH OH decarboxylation OH keto/enol tautomerization to form an aldehyde NAD+ oxidation carbonyl (hydration) (elimination reaction) (addition reaction) 7. OH OH O keto/enol tautomerization to form an alpha keto acid OH OH reaction with TPP ylid (addition reaction) OH decarboxylation to TPP enamine elimination reaction to form new 6C carbohdrate and TPP ylid TPP enamine reaction with 2C carbohydrate O H OH (addition reaction) 8. OH O O R reverse Claisen forward Claisen O (acyl substitution reaction) OH (acyl substitution reaction) 9. OH OH O NADH reduction NAD+ oxidation to an aldehyde H OH OH (addition reaction) y:\files\classes\Organic Hunger Games\Bio-Org Game newer.doc (elimination reaction) 28 10. H O HO O HO acetal formation (2 steps) O (addition reaction) (elimination reaction) OH R H overall = SN1 intermediate 11. O R HO O HO acetal hydrolysis to hemiacetal (2 steps) H (elimination reaction) OH H O intermediate (addition reaction) 12. Vit B-6 imine formation (2 steps) O OH (addition reaction) O (elimination reaction) 13. O OH N keto/enol tautomerization to new imine hydrolysis of imine to amino acid and the aldehyde version of Vit B-6 (2 steps) (addition and elimination reactions) N H 14. H OH N O H H OH R imine formation (2 steps) (addition and elimination reactions) y:\files\classes\Organic Hunger Games\Bio-Org Game newer.doc NADH reduction to an amine (addition reaction) overall = SN1 29 15. R H N OH NAD+ oxidation to an imine imine hydrolysis to an aldehyde (2 steps) (elimination reaction) (addition and elimination reactions) H OH 16. H O O O P PP O ATP O OH phosphorylation of 3o alcohol withATP (acyl-like substitution reaction) 17. O O elimination reaction to form an alkene alcohol (show as an E2 reaction) P O O OH H 18. O O P O O hydrolysis of phosphate ester to di-alcohol OH (acyl-like substitution reaction) H 19. O O O H P PP O ATP O formation of mixed anhydride (acyl-like substitution reaction) O 20. O O P O Claisen condensation O O H OH Co-A S y:\files\classes\Organic Hunger Games\Bio-Org Game newer.doc (acyl-like substitution reaction) 30 21. O O Co-A NADH reduction of keto group (addition reaction) S 22. H O O Co-A reverse Michael reaction S (elimination reaction) 23. O Co-A S NADH hydride reduction of the conjugated C=C by a Michael reaction (addition reaction) y:\files\classes\Organic Hunger Games\Bio-Org Game newer.doc 31 Methylates choline, norepinephrine, DNA (epigenetics) Biotin (carboxylations) Lipoic acid (acyl transfer) Possible reactions Aldol reverse Aldol Hemi-acetal formation reverse hemi-acetal reaction (includes ketal reactions) Acetal formation reverse acetal reaction (includes ketal reactions) Michael reaction reverse Michael reaction Carbonyl hydration reaction carbonyl dehydration reaction Tautomeric changes (keto enol and/or enol keto) (enamine chem.?) DNA / RNA base synthesis and degradation Additional possibilities??? Enamine chemistry Imine chemistry Amine oxidation to carbonyl Phosphate ester / anhydride synthesis and hydrolysis (tyrosine, serine, ATP, throxine, etc.) xxxxxxxxxx Lipid chemistry – glycerol esters, ethers, phosphates, carbohydrates y:\files\classes\Organic Hunger Games\Bio-Org Game newer.doc 32 B Forward Direction B H H H B H H O O C C alcohol aldehyde or ketone B R H O O B H R O O O O O add ROH lose H2O hemiacetal / hemiketal acetal / ketal intermediate Reverse Direction H B H B B R B O O O H B H H O O O lose ROH add H2O acetal / ketal H O O C C alcohol hemiacetal / hemiketal intermediate B H aldehyde or ketone B Forward Direction B H H H B H H O O C C alcohol aldehyde or ketone B O O R H O B H O O lose H2O hemiacetal / hemiketal intermediate add ROH R O O acetal / ketal B Reverse Direction H B H R B B B O H H H O O O O O lose ROH acetal / ketal add H2O hemiacetal / hemiketal intermediate H H O O C C alcohol y:\files\classes\Organic Hunger Games\Bio-Org Game newer.doc B aldehyde or ketone 33 Problem - What kind of reaction occurred in each part? Use a simplistic mechanism to show how the reaction could have proceeded. The following problems are more limited questions from the original “carbohydrate game” and do not include many of the biochemical “co-factors”. OH O O O 1? OH OH H OH OH O O O OH OH OH OH 2? H OH OH OH OH OH OH OH OH O OH OH OH O O OH OH O 3? OH OH HO HO OH OH OH HO OH OH OH OH HO OH OH O OH OH OH O OH 6? OH OH OH HO 5? OH O OH OH O HO 4? OH O OH 7? OH OH OH O OH H 8? OH O OH OH O 9? OH O H 12 ? (2 steps) O y:\files\classes\Organic Hunger Games\Bio-Org Game newer.doc OH 11 ? H O O 10 ? O 34 O OH OH OH OH H OH OH 13 ? OH OH OH OH OH 2 rotations 14 ? (2 steps) OH O O OH O HO OH OH O H H O O OH 18 ? HO HO O O O O OH 17 ? HO O O 15 ? H OH OH 16 ? HO OH O O 1. reverse aldol 7. keto/enol tautomerization 13. 2 x keto/enol tautomerization 2. forward aldol 8. reverse aldol 14. reverse Michael reaction 3. hemi-ketal formation to 6 atom ring 9. keto/enol tautomerization 4. reverse of hemi-ketal to 5 atom ring 10. structure shown below 5. reverse of hemi-ketal to form open chain 11. keto/enol tautomerization 15. intramolecular Michael reaction using the OH of an alcohol group 16. reverse Michael on the other side of the ring 17. keto/enol tautomerization 6. reverse Michael reaction 12. 2 x keto/enol tautomerization 18. reverse Michael reaction OH OH O enol at both ends of the double bond, can form a carbonyl on either side, one as a ketone and one as an aldehyde Problem – Use B-H+ / B: to accomplish the following transformation using simplistic mechanisms (you do not need to show lone pairs of electrons and you can combine multiple steps using several arrows). OH OH OH dehydration (reverse Michael) OH OH OH O OH OH reverse aldol to 3C aldehyde and 4 carbon 2,3-diketo structure OH OH dehydration (reverse Michael) OH keto/enol to form 1,2-diketo structure O OH y:\files\classes\Organic Hunger Games\Bio-Org Game newer.doc keto/enol to form 3,4-diketo structure reverse aldol to 1C aldehyde and 6 carbon 2,3-diketo structure 35 OH OH OH hemi-acetal formation to 5 atom ring OH OH OH O OH OH OH hemi-acetal formation to 6 atom ring OH OH O OH acetal formation with ROH OH OH O H OH acetal formation with ROH OH dehydration to form carbonyl keto/enol to form 2-keto structure O H H O HO O OH hemi-acetal ring opening HO OH O HO Michael addition of water (hydration) O cyclic ester ring opening addition of water (hydration) OH OH OH OH OH reverse aldol OH O forward aldol (reverse step) O H OH OH OH OH hemi-ketal formation to 6 atom ring O OH O H y:\files\classes\Organic Hunger Games\Bio-Org Game newer.doc reverse reaction back to carbonyl and alcohol carbonyl hydration 36 OH OH OH HO H dehydration (reverse Michael) O OH hydration (Michael) O H OH OH OH OH H H OH OH OH OH OH OH OH keto/enol tautomerization (form a ketone) (2 steps) OH OH reverse aldol to form a 3 carbon ketone and 4 carbon aldehyde O OH hydration of carbonyl OH reverse aldol O HO H OH OH keto/enol tautomerization (form an aldehyde) (2 steps) O y:\files\classes\Organic Hunger Games\Bio-Org Game newer.doc dehydration of carbonyl hydrate 37 A few possible answers OH OH OH OH OH OH OH H reverse aldol OH O O B H OH OH O B OH OH B OH B OH hemiacetal formation (6 atom ring) B B H H dehydration (reverse Michael) OH OH OH O H OH O OH O OH OH OH O H B OH OH OH OH B OH OH H OH O OH O B OH HO H reverse reaction back to carbonyl & alcohol OH B OH OH OH O H OH H O H OH O O B H OH OH forward aldol (reverse steps) H OH H OH H OH HO H hydration (Michael) OH O OH OH O OH O H OH B B OH OH OH OH OH H H OH OH B O keto/enol tautomerization (form an aldehyde) OH OH OH O H OH OH keto/enol tautomerization (form a new ketone) OH OH OH OH OH O OH OH OH OH OH OH OH OH O B H OH H OH B H y:\files\classes\Organic Hunger Games\Bio-Org Game newer.doc OH B H OH OH O OH OH OH OH reverse reaction dehydration of OH diol OH OH B OH hydration of carbonyl OH OH B B H OH OH O H H OH O OH B H B OH HO H OH H OH B H OH H O O OH OH H H B OH OH O 38 Problem - State what type of transformation occurred and show a simplistic arrow pushing mechanism for how it occurred, adding in any B: and/or B-H+ that is necessary. (an older problem set) OH a. OH OH OH O H H O OH O H H H O OH b. OH O OH OH OH OH OH OH O O OH OH OH HO O c. O OH OH OH OH OH O OH HO OH OH OH OH O OH OH OH OH H OH OH d. OH OH OH OH OH OH H O OH O OH OH HO OH OH OH O OH HO O OH OH OH OH OH OH H OH OH H O H O O H O O HO H HO HO OH O H OH R O HO OH O HO OH OH OH H H O O y:\files\classes\Organic Hunger Games\Bio-Org Game newer.doc O H OH OH OH OH HO O R HO OH OH OH O OH intermediate OH O HO OH O OH HO OH intermediate O HO R O H HO OH HO O O HO H HO R R OH HO intermediate OH R O HO O OH OH HO O OH HO HO HO OH O O g. OH H OH HO OH O O OH OH H HO O i. O OH OH H h. O O O OH OH HO OH e. OH f. O OH HO O O O OH OH HO HO HO OH OH OH O OH OH OH H O HO OH H O O 39 Partial Answers y:\files\classes\Organic Hunger Games\Bio-Org Game newer.doc 40 OH a. OH OH OH O H H O OH b. OH reverse Michael OH OH OH tautomerism O OH O OH c. Michael OH OH OH O OH OH O O OH OH OH HO hemi-acetal formation OH OH H reverse aldol OH HO O O H H OH OH O OH OH O OH OH OH OH H OH OH d. tautomerism OH OH OH OH OH OH OH O O OH reverse Michael OH OH O OH O OH HO O OH OH OH OH HO aldol O OH OH reverse aldol OH HO O OH hemi-acetal formation OH OH OH OH OH OH R O H O H OH O O O HO OH reverse acetal formation (2 steps) OH intermediate OH OH O O O y:\files\classes\Organic Hunger Games\Bio-Org Game newer.doc OH intermediate HO HO O H carbonyl hydration O R O H R O H O OH HO R O HO acetal formation HO (2 steps) O OH HO acetal formation (2 steps) OH HO OH H H reverse OH aldol O HO H R OH HO OH OH HO O OH HO intermediate H reverse acetal formation (2 steps) HO O OH acetal formation (2 steps) HO HO H O OH R O HO O OH HO OH HO HO hemi-acetal formation O acetal OH formation (2 steps) HO O OH H O g. OH H OH HO OH OH H O i. O tautomerism O O dehydration H h. HO OH e. OH f. O OH HO O H O OH OH HO HO HO tautomerism OH reverse hemi-acetal formation OH OH O OH OH OH OH O OH OH OH H O HO OH H carbonyl dehydration O O