Chapter 9
... Comparing Fermentation with Anaerobic and Aerobic Respiration • All use glycolysis (net ATP = 2) to oxidize glucose and harvest chemical energy of food • In all three, NAD+ is the oxidizing agent that accepts electrons during glycolysis • The processes have different final electron acceptors: an or ...
... Comparing Fermentation with Anaerobic and Aerobic Respiration • All use glycolysis (net ATP = 2) to oxidize glucose and harvest chemical energy of food • In all three, NAD+ is the oxidizing agent that accepts electrons during glycolysis • The processes have different final electron acceptors: an or ...
userfiles/153/my files/09_lecture_presentation 2015?id=1069
... All use glycolysis (net ATP = 2) to oxidize glucose and harvest chemical energy of food In all three, NAD+ is the oxidizing agent that accepts electrons during glycolysis BUT, they have different mechanisms for oxidizing NADH: In fermentation, an organic molecule (such as pyruvate or acetald ...
... All use glycolysis (net ATP = 2) to oxidize glucose and harvest chemical energy of food In all three, NAD+ is the oxidizing agent that accepts electrons during glycolysis BUT, they have different mechanisms for oxidizing NADH: In fermentation, an organic molecule (such as pyruvate or acetald ...
Chapter 9
... • In lactic acid fermentation, pyruvate is reduced to NADH, forming lactate as an end product, with no release of CO2 • Lactic acid fermentation by some fungi and bacteria is used to make cheese and yogurt • Human muscle cells use lactic acid fermentation to generate ATP when O2 is ...
... • In lactic acid fermentation, pyruvate is reduced to NADH, forming lactate as an end product, with no release of CO2 • Lactic acid fermentation by some fungi and bacteria is used to make cheese and yogurt • Human muscle cells use lactic acid fermentation to generate ATP when O2 is ...
lecture1
... Stage 1: The nutrient macro-molecules are broken down into their respective building blocks – Proteins will yield amino acids, polysaccharides give rise to carbohydrate units that are convertible to glucose and lipids are broken down into glycerol and fatty acids and other components. In stage II of ...
... Stage 1: The nutrient macro-molecules are broken down into their respective building blocks – Proteins will yield amino acids, polysaccharides give rise to carbohydrate units that are convertible to glucose and lipids are broken down into glycerol and fatty acids and other components. In stage II of ...
Pdf - Text of NPTEL IIT Video Lectures
... new are coupling up this electron transport chain and oxidative phosphorylation then, we can find that energy is released when electrons are transported from higher energy. That is NADH or FADH 2 to lower oxygen, lower energy oxygen. The energy is used to phosphorylate ADP. Coupling of ATP synthesis ...
... new are coupling up this electron transport chain and oxidative phosphorylation then, we can find that energy is released when electrons are transported from higher energy. That is NADH or FADH 2 to lower oxygen, lower energy oxygen. The energy is used to phosphorylate ADP. Coupling of ATP synthesis ...
Slide 1
... • Combination of amino acids into a single protein • Synthesis of RNA or DNA • Reduction of carbon-containing molecules to form hydrocarbon chain in lipid molecules • Movement of flagella • Separation of chromosomes during mitosis ...
... • Combination of amino acids into a single protein • Synthesis of RNA or DNA • Reduction of carbon-containing molecules to form hydrocarbon chain in lipid molecules • Movement of flagella • Separation of chromosomes during mitosis ...
Chapter 13 - Arcanum
... • Consumers are also called heterotrophs because they feed off of different things. ...
... • Consumers are also called heterotrophs because they feed off of different things. ...
What happened to my cousin Patrick O’Neill?
... CQ4: What would happen if Patrick lost his ability to make ATP? A: His muscles would not be able to contract. B: His neurons would not be able to conduct electrical signals. C: Both A and B. ...
... CQ4: What would happen if Patrick lost his ability to make ATP? A: His muscles would not be able to contract. B: His neurons would not be able to conduct electrical signals. C: Both A and B. ...
Bioenergetics Objectives Objectives
... • ETC chain results in pumping of H+ ions across inner mitochondrial membrane ...
... • ETC chain results in pumping of H+ ions across inner mitochondrial membrane ...
Environmental Microbiology Learning Questions
... 5. What are the limitations of fermentation? Fermenting organisms need to release electrons from the oxidative branch of substrate utilization (catabolism) on a redox potential that allows for the reaction. Thus, hydrogen partial pressures play an important role for many types of fermentation. E.g. ...
... 5. What are the limitations of fermentation? Fermenting organisms need to release electrons from the oxidative branch of substrate utilization (catabolism) on a redox potential that allows for the reaction. Thus, hydrogen partial pressures play an important role for many types of fermentation. E.g. ...
2 Pyruvic Acid
... Negative feedback prevents too much product from being produced. The product of the metabolic pathway often inhibits the rate-limiting enzyme. ...
... Negative feedback prevents too much product from being produced. The product of the metabolic pathway often inhibits the rate-limiting enzyme. ...
Citric Acid Cycle
... • The reaction is by the same mechanism as pyruvate dehydrogenase. • The same kind of large complex with E1, E2 and E3 is used. – E3 (dihyrolipoyl dehydrogenase is identical in the two complexes. ...
... • The reaction is by the same mechanism as pyruvate dehydrogenase. • The same kind of large complex with E1, E2 and E3 is used. – E3 (dihyrolipoyl dehydrogenase is identical in the two complexes. ...
"Fermentation Pathways". In: Microbial Physiology (Fourth Edition)
... The fermentation products formed by yeast can also be altered drastically through metabolic engineering. For example, introduction of a lactate dehydrogenase gene from bovine muscle (LDH-A) into S. cerevisiae engenders the production of lactic acid at levels rivaling those achieved by lactic acid ba ...
... The fermentation products formed by yeast can also be altered drastically through metabolic engineering. For example, introduction of a lactate dehydrogenase gene from bovine muscle (LDH-A) into S. cerevisiae engenders the production of lactic acid at levels rivaling those achieved by lactic acid ba ...
chapter 9 cellular respiration: harvesting
... • REDOX reactions in respiration – release energy as breakdown organic molecules • break C-C bonds • strip off electrons from C-H bonds by removing H atoms – C6H12O6 CO2 = the fuel has been oxidized • electrons attracted to more electronegative atoms – in biology, the most electronegative atom? – ...
... • REDOX reactions in respiration – release energy as breakdown organic molecules • break C-C bonds • strip off electrons from C-H bonds by removing H atoms – C6H12O6 CO2 = the fuel has been oxidized • electrons attracted to more electronegative atoms – in biology, the most electronegative atom? – ...
Correlation - EngineeringDuniya.com
... three stages of cellular respiration. Stage 1: oxidation of fatty acids,glucose, and some amino acids yields acetyl-CoA. Stage 2: oxidation of acetyl groups in the citric acid cycle includes four steps in which electrons are abstracted. Stage 3: electrons carried by NADH andFADH2 are funneled into a ...
... three stages of cellular respiration. Stage 1: oxidation of fatty acids,glucose, and some amino acids yields acetyl-CoA. Stage 2: oxidation of acetyl groups in the citric acid cycle includes four steps in which electrons are abstracted. Stage 3: electrons carried by NADH andFADH2 are funneled into a ...
Chapter 19
... In most animals, acetyl CoA is NOT a carbon source for the net formation of glucose (2 carbons of acetyl CoA enter CA cycle, 2 carbons are released as 2 CO2 ...
... In most animals, acetyl CoA is NOT a carbon source for the net formation of glucose (2 carbons of acetyl CoA enter CA cycle, 2 carbons are released as 2 CO2 ...
Harvesting Electrons from the Citric Acid Cycle
... In most animals, acetyl CoA is NOT a carbon source for the net formation of glucose (2 carbons of acetyl CoA enter CA cycle, 2 carbons are released as 2 CO2 ...
... In most animals, acetyl CoA is NOT a carbon source for the net formation of glucose (2 carbons of acetyl CoA enter CA cycle, 2 carbons are released as 2 CO2 ...
Chapter 15 The Tricarboxylic Acid Cycle
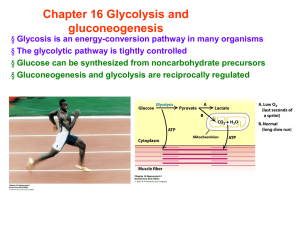
... The components of respiratory metabolism : 4 events for glucose catabolism : 1) Glycolytic pathway : production of pyruvate from glucose (in cytosol) 2) TCA cycle : oxidation of pyruvate to generate reduced electrons (coenzymes) (mitochondrial matrix) 3) Electron-transport chain : reoxidation of coe ...
... The components of respiratory metabolism : 4 events for glucose catabolism : 1) Glycolytic pathway : production of pyruvate from glucose (in cytosol) 2) TCA cycle : oxidation of pyruvate to generate reduced electrons (coenzymes) (mitochondrial matrix) 3) Electron-transport chain : reoxidation of coe ...
Lec 3: Carbohydrate metabolism
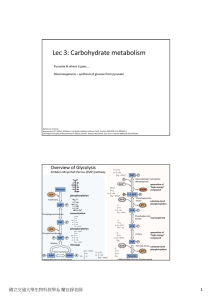
... (Step 7), all the other steps associated with ATP consumption or generation are regulated steps in the pathway. Why? These reactions have large decrease in ΔG, which makes them irreversible steps in vivo. Recall that irreversible steps are the place for metabolic control!! ...
... (Step 7), all the other steps associated with ATP consumption or generation are regulated steps in the pathway. Why? These reactions have large decrease in ΔG, which makes them irreversible steps in vivo. Recall that irreversible steps are the place for metabolic control!! ...
Section 3-1 and Section 3-2 Book Work Review – Finding the Good
... 18. What is an ecological pyramid? ANSWER: A diagram that shows the relative amounts of energy or matter contained within each trophic level in a food chain or food web. Each time you rise a level on an energy pyramid, what number do you divide the energy by? ANSWER: 10! ...
... 18. What is an ecological pyramid? ANSWER: A diagram that shows the relative amounts of energy or matter contained within each trophic level in a food chain or food web. Each time you rise a level on an energy pyramid, what number do you divide the energy by? ANSWER: 10! ...
- Catalyst
... Step 1) Write the half-reactions for the chemical equation. Step 2) For each reaction, balance the atoms other than O and H. Step 3) Add H2O to balance O, then H+ to balance H. Step 4) Balance the charge by adding electrons. The net charge of the reactants should equal the net charge of the products ...
... Step 1) Write the half-reactions for the chemical equation. Step 2) For each reaction, balance the atoms other than O and H. Step 3) Add H2O to balance O, then H+ to balance H. Step 4) Balance the charge by adding electrons. The net charge of the reactants should equal the net charge of the products ...
H + - WordPress.com
... Generation of a pH gradient ([H+]) and charge difference (negative in the matrix) across the inner membrane constitute the protonmotive force that can be used to drive ATP synthesis and transport processes. ...
... Generation of a pH gradient ([H+]) and charge difference (negative in the matrix) across the inner membrane constitute the protonmotive force that can be used to drive ATP synthesis and transport processes. ...
Microbial metabolism
Microbial metabolism is the means by which a microbe obtains the energy and nutrients (e.g. carbon) it needs to live and reproduce. Microbes use many different types of metabolic strategies and species can often be differentiated from each other based on metabolic characteristics. The specific metabolic properties of a microbe are the major factors in determining that microbe’s ecological niche, and often allow for that microbe to be useful in industrial processes or responsible for biogeochemical cycles.== Types of microbial metabolism ==All microbial metabolisms can be arranged according to three principles:1. How the organism obtains carbon for synthesising cell mass: autotrophic – carbon is obtained from carbon dioxide (CO2) heterotrophic – carbon is obtained from organic compounds mixotrophic – carbon is obtained from both organic compounds and by fixing carbon dioxide2. How the organism obtains reducing equivalents used either in energy conservation or in biosynthetic reactions: lithotrophic – reducing equivalents are obtained from inorganic compounds organotrophic – reducing equivalents are obtained from organic compounds3. How the organism obtains energy for living and growing: chemotrophic – energy is obtained from external chemical compounds phototrophic – energy is obtained from lightIn practice, these terms are almost freely combined. Typical examples are as follows: chemolithoautotrophs obtain energy from the oxidation of inorganic compounds and carbon from the fixation of carbon dioxide. Examples: Nitrifying bacteria, Sulfur-oxidizing bacteria, Iron-oxidizing bacteria, Knallgas-bacteria photolithoautotrophs obtain energy from light and carbon from the fixation of carbon dioxide, using reducing equivalents from inorganic compounds. Examples: Cyanobacteria (water (H2O) as reducing equivalent donor), Chlorobiaceae, Chromatiaceae (hydrogen sulfide (H2S) as reducing equivalent donor), Chloroflexus (hydrogen (H2) as reducing equivalent donor) chemolithoheterotrophs obtain energy from the oxidation of inorganic compounds, but cannot fix carbon dioxide (CO2). Examples: some Thiobacilus, some Beggiatoa, some Nitrobacter spp., Wolinella (with H2 as reducing equivalent donor), some Knallgas-bacteria, some sulfate-reducing bacteria chemoorganoheterotrophs obtain energy, carbon, and reducing equivalents for biosynthetic reactions from organic compounds. Examples: most bacteria, e. g. Escherichia coli, Bacillus spp., Actinobacteria photoorganoheterotrophs obtain energy from light, carbon and reducing equivalents for biosynthetic reactions from organic compounds. Some species are strictly heterotrophic, many others can also fix carbon dioxide and are mixotrophic. Examples: Rhodobacter, Rhodopseudomonas, Rhodospirillum, Rhodomicrobium, Rhodocyclus, Heliobacterium, Chloroflexus (alternatively to photolithoautotrophy with hydrogen)