
21/Interdependence in the Sea
... IDENTIFY important nutrient cycles in the ocean environment. EXPLAIN characteristics of oceanic food chains and food webs. DISCUSS the importance of symbiotic relationships in the sea. DESCRIBE how succession occurs in marine environments. ...
... IDENTIFY important nutrient cycles in the ocean environment. EXPLAIN characteristics of oceanic food chains and food webs. DISCUSS the importance of symbiotic relationships in the sea. DESCRIBE how succession occurs in marine environments. ...
Sept14
... reaction know as dehydration synthesis to make polymers. The reverse reaction of breaking up polymers is accomplished by another chemical reaction known as hydrolysis. The following animation's illustrate these reactions http://science.nhmccd.edu/biol/dehydrat/dehydrat.html ...
... reaction know as dehydration synthesis to make polymers. The reverse reaction of breaking up polymers is accomplished by another chemical reaction known as hydrolysis. The following animation's illustrate these reactions http://science.nhmccd.edu/biol/dehydrat/dehydrat.html ...
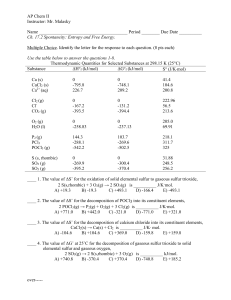
AP Chem II Instructor: Mr. Malasky Name Period ______ Due Date
... ____ 5. The value of ΔG˚ at 25˚C for the decomposition of gaseous sulfur dioxide to solid elemental sulfur and gaseous oxygen, SO2(g) → 2 S (s,rhombic) + O2(g) is __________ kJ/mol. A) +395.2 B) +269.9 C) -269.9 D) +300.4 E) -300.4 ____ 6. The value of ΔG˚ at 25˚C for the formation of POCl3 from it ...
... ____ 5. The value of ΔG˚ at 25˚C for the decomposition of gaseous sulfur dioxide to solid elemental sulfur and gaseous oxygen, SO2(g) → 2 S (s,rhombic) + O2(g) is __________ kJ/mol. A) +395.2 B) +269.9 C) -269.9 D) +300.4 E) -300.4 ____ 6. The value of ΔG˚ at 25˚C for the formation of POCl3 from it ...
Chapter 2 The Chemistry of Life Worksheets
... A compound found mainly in living things is known as an organic compound. Organic compounds make up the cells and other structures of organisms and carry out life processes. Carbon is the main element in organic compounds, so carbon is essential to life on Earth. Without carbon, life as we know it c ...
... A compound found mainly in living things is known as an organic compound. Organic compounds make up the cells and other structures of organisms and carry out life processes. Carbon is the main element in organic compounds, so carbon is essential to life on Earth. Without carbon, life as we know it c ...
Alcohol Metabolism
... Pyruvate and acetyl CoA accumulate Acetyl CoA fatty acid synthesis FAs accumulate in the liver Once the liver is congested with fat: Decreased efficiency of hepatocytes Decreased conversion of vitamin D to active form Decreased gluconeogenesis Decreased glu & increased acetyl CoA ketosis ...
... Pyruvate and acetyl CoA accumulate Acetyl CoA fatty acid synthesis FAs accumulate in the liver Once the liver is congested with fat: Decreased efficiency of hepatocytes Decreased conversion of vitamin D to active form Decreased gluconeogenesis Decreased glu & increased acetyl CoA ketosis ...
Kevin Ahern's Biochemistry Course (BB 350) at Oregon State University
... two enzymes are enoyl-CoA isomerase and 2,4 dienoyl-CoA reductase (also known as Dina). The first enzyme catalyzes conversion of cis bonds between carbons 3 and 4 to trans bonds between carbons 2 and 3 so it can be oxidized in beta oxidation. Dina catalyzes conversion of two double bonds into one ci ...
... two enzymes are enoyl-CoA isomerase and 2,4 dienoyl-CoA reductase (also known as Dina). The first enzyme catalyzes conversion of cis bonds between carbons 3 and 4 to trans bonds between carbons 2 and 3 so it can be oxidized in beta oxidation. Dina catalyzes conversion of two double bonds into one ci ...
Slide 1
... homeostasis, to move, and to reproduce • Photosynthesis converts energy from the sun to glucose and O2 • Cellular respiration breaks down glucose and releases energy in ATP • Energy flows through an ecosystem; chemicals are recycled Copyright © 2005 Pearson Education, Inc. Publishing as Benjamin Cum ...
... homeostasis, to move, and to reproduce • Photosynthesis converts energy from the sun to glucose and O2 • Cellular respiration breaks down glucose and releases energy in ATP • Energy flows through an ecosystem; chemicals are recycled Copyright © 2005 Pearson Education, Inc. Publishing as Benjamin Cum ...
Printer-friendly Version
... and brings waters close to equilibrium). Assuming an annual average water temperature of 14◦ C, the O2 saturation level (atmospheric equilibrium) is about 320 µM, while annual averages of O2 concentration in the late 2000’s are well below, at about 200 µM as shown in figure 2. This means that either ...
... and brings waters close to equilibrium). Assuming an annual average water temperature of 14◦ C, the O2 saturation level (atmospheric equilibrium) is about 320 µM, while annual averages of O2 concentration in the late 2000’s are well below, at about 200 µM as shown in figure 2. This means that either ...
ATP ENERGY PRODUCTION - SHMD 339: Exercise Physiology 3
... 1. The phosphocreatine system (ATP/PC) or alactic system. 2. The lactic acid system or anaerobic glycolysis. 3. The aerobic system. • Each method is good at supplying energy for particular energy demands and duration. • Systems 1 & 2 are anaerobic = take place without ...
... 1. The phosphocreatine system (ATP/PC) or alactic system. 2. The lactic acid system or anaerobic glycolysis. 3. The aerobic system. • Each method is good at supplying energy for particular energy demands and duration. • Systems 1 & 2 are anaerobic = take place without ...
Cellular Respiration
... Cellular Respiration • A catabolic, exergonic, oxygen (O2) requiring process that uses energy extracted from macromolecules (glucose) to produce energy (ATP) and water (H2O). ...
... Cellular Respiration • A catabolic, exergonic, oxygen (O2) requiring process that uses energy extracted from macromolecules (glucose) to produce energy (ATP) and water (H2O). ...
Topic 14 - Fertilisers
... However, nitrogen is un-reactive but not inert. This means it is difficult getting it to react but it can be done (using electricity i.e. lightening or a spark plug). The nitrogen will form oxides which dissolve in water forming acids. Nitrogen dioxide, a brown gas, can be made when air (21% oxygen ...
... However, nitrogen is un-reactive but not inert. This means it is difficult getting it to react but it can be done (using electricity i.e. lightening or a spark plug). The nitrogen will form oxides which dissolve in water forming acids. Nitrogen dioxide, a brown gas, can be made when air (21% oxygen ...
ATP - Luzzago
... Catabolic Pathways and Production of ATP • Fermentation is a partial degradation of sugars, to produce ATP, that occurs without O2 • Aerobic respiration consumes organic molecules and O2 and yields ATP • Anaerobic respiration is similar to aerobic respiration but consumes compounds other than O2 ...
... Catabolic Pathways and Production of ATP • Fermentation is a partial degradation of sugars, to produce ATP, that occurs without O2 • Aerobic respiration consumes organic molecules and O2 and yields ATP • Anaerobic respiration is similar to aerobic respiration but consumes compounds other than O2 ...
Ecology Review Science Department
... 28. What factors can affect the functioning of an enzyme? Change in: pH Temperature Salinity (ions) Substrate (starting ingredient) concentration ...
... 28. What factors can affect the functioning of an enzyme? Change in: pH Temperature Salinity (ions) Substrate (starting ingredient) concentration ...
Cell Respiration
... As long as food molecules are available to be converted into glucose, a cell can produce ATP. Continual production creates NADH accumulation and NAD+ depletion. NADH must be recycled into NAD+. • Aerobic respiration - oxygen as electron acceptor • Fermentation - organic molecule ...
... As long as food molecules are available to be converted into glucose, a cell can produce ATP. Continual production creates NADH accumulation and NAD+ depletion. NADH must be recycled into NAD+. • Aerobic respiration - oxygen as electron acceptor • Fermentation - organic molecule ...
The bioinorganic chemistry of the ancient ocean: The co
... • Stromatolites: do not require photosynthesis do be produced (early ocean much closer to saturation, methane oxidizers can produce ...
... • Stromatolites: do not require photosynthesis do be produced (early ocean much closer to saturation, methane oxidizers can produce ...
[j26]Chapter 5#
... ___ 15. It is common for certain tissues like skeletal muscle to derive energy (ATP) from anaerobic respiration without permanent injury or damage to the tissue. ...
... ___ 15. It is common for certain tissues like skeletal muscle to derive energy (ATP) from anaerobic respiration without permanent injury or damage to the tissue. ...
Honors Marine Biology
... • Even though this zone is small, it has the greatest variation of environmental factors as compared to any other marine ecosystem. • There are even several differences within a single square meter containing huge diversities of life. ...
... • Even though this zone is small, it has the greatest variation of environmental factors as compared to any other marine ecosystem. • There are even several differences within a single square meter containing huge diversities of life. ...
The rocky roots of the acetyl
... with an estimated standard free energy (DG00 ) of 2 59 kJ mol21 [36], which is sufficient to drive ATP synthesis [37]. In a nutshell, the pathway reduces CO2 to form an energy-rich thioester in the presence of a thiol with the help of electrons supplied by H2, while releasing enough energy to make A ...
... with an estimated standard free energy (DG00 ) of 2 59 kJ mol21 [36], which is sufficient to drive ATP synthesis [37]. In a nutshell, the pathway reduces CO2 to form an energy-rich thioester in the presence of a thiol with the help of electrons supplied by H2, while releasing enough energy to make A ...
Exam 3 Study Guide
... Bring your student ID. Failure to do so will result in getting your exam back later. You may use a NON-PROGRAMMABLE calculator. All papers, books, phones, and electronic devices must be in a sealed bag under your seat. Exam Content: The exam will cover chapters 15-21 and 24-26. (Chapter 26 wil ...
... Bring your student ID. Failure to do so will result in getting your exam back later. You may use a NON-PROGRAMMABLE calculator. All papers, books, phones, and electronic devices must be in a sealed bag under your seat. Exam Content: The exam will cover chapters 15-21 and 24-26. (Chapter 26 wil ...
Microbial metabolism
Microbial metabolism is the means by which a microbe obtains the energy and nutrients (e.g. carbon) it needs to live and reproduce. Microbes use many different types of metabolic strategies and species can often be differentiated from each other based on metabolic characteristics. The specific metabolic properties of a microbe are the major factors in determining that microbe’s ecological niche, and often allow for that microbe to be useful in industrial processes or responsible for biogeochemical cycles.== Types of microbial metabolism ==All microbial metabolisms can be arranged according to three principles:1. How the organism obtains carbon for synthesising cell mass: autotrophic – carbon is obtained from carbon dioxide (CO2) heterotrophic – carbon is obtained from organic compounds mixotrophic – carbon is obtained from both organic compounds and by fixing carbon dioxide2. How the organism obtains reducing equivalents used either in energy conservation or in biosynthetic reactions: lithotrophic – reducing equivalents are obtained from inorganic compounds organotrophic – reducing equivalents are obtained from organic compounds3. How the organism obtains energy for living and growing: chemotrophic – energy is obtained from external chemical compounds phototrophic – energy is obtained from lightIn practice, these terms are almost freely combined. Typical examples are as follows: chemolithoautotrophs obtain energy from the oxidation of inorganic compounds and carbon from the fixation of carbon dioxide. Examples: Nitrifying bacteria, Sulfur-oxidizing bacteria, Iron-oxidizing bacteria, Knallgas-bacteria photolithoautotrophs obtain energy from light and carbon from the fixation of carbon dioxide, using reducing equivalents from inorganic compounds. Examples: Cyanobacteria (water (H2O) as reducing equivalent donor), Chlorobiaceae, Chromatiaceae (hydrogen sulfide (H2S) as reducing equivalent donor), Chloroflexus (hydrogen (H2) as reducing equivalent donor) chemolithoheterotrophs obtain energy from the oxidation of inorganic compounds, but cannot fix carbon dioxide (CO2). Examples: some Thiobacilus, some Beggiatoa, some Nitrobacter spp., Wolinella (with H2 as reducing equivalent donor), some Knallgas-bacteria, some sulfate-reducing bacteria chemoorganoheterotrophs obtain energy, carbon, and reducing equivalents for biosynthetic reactions from organic compounds. Examples: most bacteria, e. g. Escherichia coli, Bacillus spp., Actinobacteria photoorganoheterotrophs obtain energy from light, carbon and reducing equivalents for biosynthetic reactions from organic compounds. Some species are strictly heterotrophic, many others can also fix carbon dioxide and are mixotrophic. Examples: Rhodobacter, Rhodopseudomonas, Rhodospirillum, Rhodomicrobium, Rhodocyclus, Heliobacterium, Chloroflexus (alternatively to photolithoautotrophy with hydrogen)


















![[j26]Chapter 5#](http://s1.studyres.com/store/data/009375917_1-4b76508e3cea6c5c195183cfa3853e79-300x300.png)


