A: Objective type questions: Choose the correct answers Most
... heterotrophs; chemoautotrophs c. chemoautotrophs; heterotrophs d. photoautotrophs; chemoautotrophs e. none are true. Ans. A ...
... heterotrophs; chemoautotrophs c. chemoautotrophs; heterotrophs d. photoautotrophs; chemoautotrophs e. none are true. Ans. A ...
$doc.title
... anaerobically in a validated (that is, base broth was run) PR sucrose, no color change or turbidity is observed. When a pure culture of the same bacterium is incubated anaerobically ...
... anaerobically in a validated (that is, base broth was run) PR sucrose, no color change or turbidity is observed. When a pure culture of the same bacterium is incubated anaerobically ...
TRICARBOXYLIC ACID CYCLE
... oxidation of carbohydrate, fat and amino acids via acetyl coenzyme A. • Pyruvate is converted to acetyl coenzyme A by the pyruvate dehydrogenase complex. • The reactions of the TCA cycle generate carbon dioxide, reduced NAD, reduced FAD and GTP • There are negative and positive controls for the TCA ...
... oxidation of carbohydrate, fat and amino acids via acetyl coenzyme A. • Pyruvate is converted to acetyl coenzyme A by the pyruvate dehydrogenase complex. • The reactions of the TCA cycle generate carbon dioxide, reduced NAD, reduced FAD and GTP • There are negative and positive controls for the TCA ...
Fatty Acid Catabolism - Chemistry Courses: About
... Activation of Acetyl Group • Acetyl CoA carboxylase (analogous to pyruvate carboxylase of gluconeogenesis) • Requires biotin, ATP • A regulation step—shifts fuel away from CAC ...
... Activation of Acetyl Group • Acetyl CoA carboxylase (analogous to pyruvate carboxylase of gluconeogenesis) • Requires biotin, ATP • A regulation step—shifts fuel away from CAC ...
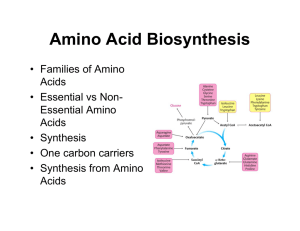
Amino Acid Biosynthesis
... Prokaryotic cells Nitrogenase reaction: N2 + 8 H+ + 8 e- + 16 ATP Æ 2 NH3 + H2 + 16 ATP + 16 Pi Ammonia assimilated in amino acids ...
... Prokaryotic cells Nitrogenase reaction: N2 + 8 H+ + 8 e- + 16 ATP Æ 2 NH3 + H2 + 16 ATP + 16 Pi Ammonia assimilated in amino acids ...
AP Biology Review Sheet for the Midterm Exam Unit 1 – Evolution
... Explain the role of cellular respiration in the catabolism of fuel molecules and release of energy for cellular work Relate glycolysis and the citric acid cycle to catabolic and anabolic processes Explain how cellular respiration controls the energy release from glucose and ultimately transfer ...
... Explain the role of cellular respiration in the catabolism of fuel molecules and release of energy for cellular work Relate glycolysis and the citric acid cycle to catabolic and anabolic processes Explain how cellular respiration controls the energy release from glucose and ultimately transfer ...
metabolism of amino acids
... • Positive nitrogen balance- an excess of ingested over excreted nitrogen- during growth and pregnancy • Negative nitrogen balance – output exceeds intakeduring surgery, advanced cancer or malnutrition ...
... • Positive nitrogen balance- an excess of ingested over excreted nitrogen- during growth and pregnancy • Negative nitrogen balance – output exceeds intakeduring surgery, advanced cancer or malnutrition ...
Oxidative Phosphorylation - Creighton Chemistry Webserver
... Cytocrome c to molecular O2 (reducing it to H2 O) ...
... Cytocrome c to molecular O2 (reducing it to H2 O) ...
2 - ATP
... Cellular Respiration • A catabolic, exergonic, oxygen (O2) requiring process that uses energy extracted from macromolecules (glucose) to produce energy (ATP) and water (H2O). ...
... Cellular Respiration • A catabolic, exergonic, oxygen (O2) requiring process that uses energy extracted from macromolecules (glucose) to produce energy (ATP) and water (H2O). ...
AQA(B) A2 Module 5: Environment Contents
... Decay (also known as decomposition, putrefaction or rotting) is the breakdown of detritus. Decay doesn't "just happen" but is brought about by living organisms collectively called decomposers. There are two groups of decomposers: Saprophytes (or decomposers) are microbes (fungi and bacteria) that li ...
... Decay (also known as decomposition, putrefaction or rotting) is the breakdown of detritus. Decay doesn't "just happen" but is brought about by living organisms collectively called decomposers. There are two groups of decomposers: Saprophytes (or decomposers) are microbes (fungi and bacteria) that li ...
Microbial alteration of stable nitrogen and carbon isotopic
... The fractionation of arginine is more complex. The presence of four separate nitrogen atoms, almost certainly with different isotopic compositions, complicates the interpretation. Preferential deamination of lighter nitrogen atoms at the same position in the compound could also explain the observati ...
... The fractionation of arginine is more complex. The presence of four separate nitrogen atoms, almost certainly with different isotopic compositions, complicates the interpretation. Preferential deamination of lighter nitrogen atoms at the same position in the compound could also explain the observati ...
File
... http://1.bp.blogspot.com/-7rJudWIXTJQ/Tt_BPza86TI/AAAAAAAAAEU/2a9QD4FEoIY/s1600/foodchain.gif ...
... http://1.bp.blogspot.com/-7rJudWIXTJQ/Tt_BPza86TI/AAAAAAAAAEU/2a9QD4FEoIY/s1600/foodchain.gif ...
Cell Energy - Kuliah FTSL
... Formation of acids (facultative anaerobic bacteria) HPO42NH4+/NH 3 H 2S ...
... Formation of acids (facultative anaerobic bacteria) HPO42NH4+/NH 3 H 2S ...
Review sheet – Chapters 13 and 14 (Plankton)
... Understand that this mass migration is referred to as the “deep scattering layer” for its ability to be detected by echo-sounding equipment as a distinct layer deep in the water column during the day ...
... Understand that this mass migration is referred to as the “deep scattering layer” for its ability to be detected by echo-sounding equipment as a distinct layer deep in the water column during the day ...
Oxidation and Reduction
... beautiful green patina as it ages. Metals rust or corrode in the presence of air and water. Minerals (ionic compounds) found in ore can be decomposed with the use of electricity to produce pure metals and nonmetals. All of these reactions are examples of oxidation and reduction, otherwise known as r ...
... beautiful green patina as it ages. Metals rust or corrode in the presence of air and water. Minerals (ionic compounds) found in ore can be decomposed with the use of electricity to produce pure metals and nonmetals. All of these reactions are examples of oxidation and reduction, otherwise known as r ...
doc BIOL210syllabus
... An overview of metabolism from the standpoint of Escherichia coli. Enzymes and the specificity of metabolic reactions Chemical equilibrium Predicting the direction of a reaction Readings: Lodish et al., pp. 49-51; Berg et al., pp. 373-375. Lecture 2. Energy in Biological Systems I. January 6 ...
... An overview of metabolism from the standpoint of Escherichia coli. Enzymes and the specificity of metabolic reactions Chemical equilibrium Predicting the direction of a reaction Readings: Lodish et al., pp. 49-51; Berg et al., pp. 373-375. Lecture 2. Energy in Biological Systems I. January 6 ...
Lecture 7: Metabolic Regulation - University of California, Berkeley
... Epinephrine & the cAMP Cascade. Epinephrine is secreted. There are many epinephrine receptors on the surface of muscle cells. Muscle is a characteristic organ where the effect of epinephrine takes place. The cyclic-AMP (cAMP) cascade begins with the production of cAMP, which is a pure signaling mole ...
... Epinephrine & the cAMP Cascade. Epinephrine is secreted. There are many epinephrine receptors on the surface of muscle cells. Muscle is a characteristic organ where the effect of epinephrine takes place. The cyclic-AMP (cAMP) cascade begins with the production of cAMP, which is a pure signaling mole ...
doc Syllabus 201
... An overview of metabolism from the standpoint of Escherichia coli. Enzymes and the specificity of metabolic reactions Chemical equilibrium Predicting the direction of a reaction Readings: Lodish et al., pp. 49-51; Berg et al., pp. 373-375. Lecture 2. Energy in Biological Systems I. January 6 ...
... An overview of metabolism from the standpoint of Escherichia coli. Enzymes and the specificity of metabolic reactions Chemical equilibrium Predicting the direction of a reaction Readings: Lodish et al., pp. 49-51; Berg et al., pp. 373-375. Lecture 2. Energy in Biological Systems I. January 6 ...
Biology 177-201B
... An overview of metabolism from the standpoint of Escherichia coli. Enzymes and the specificity of metabolic reactions Chemical equilibrium Predicting the direction of a reaction Readings: Lodish et al., pp. 49-51; Berg et al., pp. 373-375. Lecture 2. Energy in Biological Systems I. January 6 ...
... An overview of metabolism from the standpoint of Escherichia coli. Enzymes and the specificity of metabolic reactions Chemical equilibrium Predicting the direction of a reaction Readings: Lodish et al., pp. 49-51; Berg et al., pp. 373-375. Lecture 2. Energy in Biological Systems I. January 6 ...
Answer Set 2
... 3. a. When [S+] is much greater than the value of KM, pH will have a negligible effect on the enzyme because S+ will interact with E- as soon as the enzyme becomes available. b. When [S+] is much less than the value of KM, the plot of Vo versus pH becomes essentially a titration curve for the ioniza ...
... 3. a. When [S+] is much greater than the value of KM, pH will have a negligible effect on the enzyme because S+ will interact with E- as soon as the enzyme becomes available. b. When [S+] is much less than the value of KM, the plot of Vo versus pH becomes essentially a titration curve for the ioniza ...
Teaching metabolic pathways
... Oxidation/reduction reactions are also important in the energy profile of a pathway, since the energy of high potential electrons can readily be converted into ATP by oxidative phosphorylation. The pathway of reducing equivalents can usually be described as the involvement of electron carriers, such ...
... Oxidation/reduction reactions are also important in the energy profile of a pathway, since the energy of high potential electrons can readily be converted into ATP by oxidative phosphorylation. The pathway of reducing equivalents can usually be described as the involvement of electron carriers, such ...
Microbial metabolism
Microbial metabolism is the means by which a microbe obtains the energy and nutrients (e.g. carbon) it needs to live and reproduce. Microbes use many different types of metabolic strategies and species can often be differentiated from each other based on metabolic characteristics. The specific metabolic properties of a microbe are the major factors in determining that microbe’s ecological niche, and often allow for that microbe to be useful in industrial processes or responsible for biogeochemical cycles.== Types of microbial metabolism ==All microbial metabolisms can be arranged according to three principles:1. How the organism obtains carbon for synthesising cell mass: autotrophic – carbon is obtained from carbon dioxide (CO2) heterotrophic – carbon is obtained from organic compounds mixotrophic – carbon is obtained from both organic compounds and by fixing carbon dioxide2. How the organism obtains reducing equivalents used either in energy conservation or in biosynthetic reactions: lithotrophic – reducing equivalents are obtained from inorganic compounds organotrophic – reducing equivalents are obtained from organic compounds3. How the organism obtains energy for living and growing: chemotrophic – energy is obtained from external chemical compounds phototrophic – energy is obtained from lightIn practice, these terms are almost freely combined. Typical examples are as follows: chemolithoautotrophs obtain energy from the oxidation of inorganic compounds and carbon from the fixation of carbon dioxide. Examples: Nitrifying bacteria, Sulfur-oxidizing bacteria, Iron-oxidizing bacteria, Knallgas-bacteria photolithoautotrophs obtain energy from light and carbon from the fixation of carbon dioxide, using reducing equivalents from inorganic compounds. Examples: Cyanobacteria (water (H2O) as reducing equivalent donor), Chlorobiaceae, Chromatiaceae (hydrogen sulfide (H2S) as reducing equivalent donor), Chloroflexus (hydrogen (H2) as reducing equivalent donor) chemolithoheterotrophs obtain energy from the oxidation of inorganic compounds, but cannot fix carbon dioxide (CO2). Examples: some Thiobacilus, some Beggiatoa, some Nitrobacter spp., Wolinella (with H2 as reducing equivalent donor), some Knallgas-bacteria, some sulfate-reducing bacteria chemoorganoheterotrophs obtain energy, carbon, and reducing equivalents for biosynthetic reactions from organic compounds. Examples: most bacteria, e. g. Escherichia coli, Bacillus spp., Actinobacteria photoorganoheterotrophs obtain energy from light, carbon and reducing equivalents for biosynthetic reactions from organic compounds. Some species are strictly heterotrophic, many others can also fix carbon dioxide and are mixotrophic. Examples: Rhodobacter, Rhodopseudomonas, Rhodospirillum, Rhodomicrobium, Rhodocyclus, Heliobacterium, Chloroflexus (alternatively to photolithoautotrophy with hydrogen)