ATP
... series of enzymes in the mitochondrial membrane 2. at the end of the chain, an enzyme combines electrons from the chain 3. with H ions from the cells fluid and O2 to form H2O. 4. O2 is the final electron acceptor, therefore O2 is necessary for obtaining E from NADH and FADH2 ...
... series of enzymes in the mitochondrial membrane 2. at the end of the chain, an enzyme combines electrons from the chain 3. with H ions from the cells fluid and O2 to form H2O. 4. O2 is the final electron acceptor, therefore O2 is necessary for obtaining E from NADH and FADH2 ...
Carefully detach the last page. It is the Data Sheet.
... incomplete chemical equation for the radioactive decay of 131I is given below. ...
... incomplete chemical equation for the radioactive decay of 131I is given below. ...
Microorganisms_Background_Info
... a bacterial cell, most of them must be broken down into simpler substances. Enzymes do this by acting as catalysts and increasing the rate of biochemical reactions. A catalyst initiates a chemical reaction but is not used up in the process. A catalyst also enables a chemical reaction to proceed unde ...
... a bacterial cell, most of them must be broken down into simpler substances. Enzymes do this by acting as catalysts and increasing the rate of biochemical reactions. A catalyst initiates a chemical reaction but is not used up in the process. A catalyst also enables a chemical reaction to proceed unde ...
chapt06HOv2.ppt
... (a) Energy is released when electrons are moved from an energy source with a low affinity for electrons to a terminal electron acceptor with a higher affinity. ...
... (a) Energy is released when electrons are moved from an energy source with a low affinity for electrons to a terminal electron acceptor with a higher affinity. ...
Lecture_4_Glycolysis
... The reaction catalyzed by the mutase involves a phosphorylated enzyme intermediate and catalytic amounts of 2, 3-bisphosphoglycerate. ...
... The reaction catalyzed by the mutase involves a phosphorylated enzyme intermediate and catalytic amounts of 2, 3-bisphosphoglycerate. ...
design of energy metabolism
... sustained anaerobic pathways and greater used of arginine phosphate (cephalopods), creatine phosphate, and lactate, with their high power output, which is necessary to fuel intense activity. AEROBIC METABOLISM – pathways are available to use carbohydrates, fats, and proteins. All substrates eventual ...
... sustained anaerobic pathways and greater used of arginine phosphate (cephalopods), creatine phosphate, and lactate, with their high power output, which is necessary to fuel intense activity. AEROBIC METABOLISM – pathways are available to use carbohydrates, fats, and proteins. All substrates eventual ...
Toxic Effects
... Lower cellular concentrations by sequestration Prevention of damage by removal of xenobiotics before they reach the site of action Molecular mechanisms of activity eg the pesticide dimilin – acts on the site of formation of chitin. Thus only affects those arthropods with chitinous exoskeleton In con ...
... Lower cellular concentrations by sequestration Prevention of damage by removal of xenobiotics before they reach the site of action Molecular mechanisms of activity eg the pesticide dimilin – acts on the site of formation of chitin. Thus only affects those arthropods with chitinous exoskeleton In con ...
Chapter 26 - McGraw Hill Higher Education
... – pump protons from matrix into space between inner and outer mitochondrial membranes – creates steep electrochemical gradient for H+ across inner mitochondrial membrane ...
... – pump protons from matrix into space between inner and outer mitochondrial membranes – creates steep electrochemical gradient for H+ across inner mitochondrial membrane ...
Chapter 8
... • Cells require a constant source of energy for life processes but keep only a small amount of ATP on hand. Cells can regenerate ATP as needed by using the energy stored in foods like glucose. • The energy stored in glucose by photosynthesis is released by cellular respiration and repackaged into t ...
... • Cells require a constant source of energy for life processes but keep only a small amount of ATP on hand. Cells can regenerate ATP as needed by using the energy stored in foods like glucose. • The energy stored in glucose by photosynthesis is released by cellular respiration and repackaged into t ...
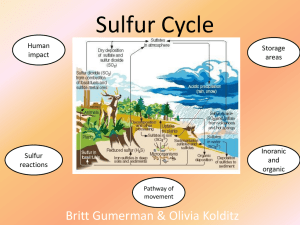
Sulfur Cycle - Walshearthsciences
... Refining sulfur-containing petroleum to make gasoline, heating oil, and other useful products. ...
... Refining sulfur-containing petroleum to make gasoline, heating oil, and other useful products. ...
Carbon Compounds slideshow Carbon Compounds
... • Atom – Basic unit of matter, smallest particle of an _______________. • Compound – Pure substance made of two or more elements, e.g. water (H2O) carbon dioxide (CO2) ______________ _______________________ atoms • Molecule – Particle made of two or more __________ joined together. compound Smallest ...
... • Atom – Basic unit of matter, smallest particle of an _______________. • Compound – Pure substance made of two or more elements, e.g. water (H2O) carbon dioxide (CO2) ______________ _______________________ atoms • Molecule – Particle made of two or more __________ joined together. compound Smallest ...
Supplemental notes in pdf
... Life on earth is made possible by the biochemical reactions of photosynthesis, carbon fixation and aerobic respiration which together convert solar energy into ATP (and NADPH) which is used to synthesize carbohydrates from CO2 and H2O. Aerobic organisms, such as ourselves, consume carbohydrates as a ...
... Life on earth is made possible by the biochemical reactions of photosynthesis, carbon fixation and aerobic respiration which together convert solar energy into ATP (and NADPH) which is used to synthesize carbohydrates from CO2 and H2O. Aerobic organisms, such as ourselves, consume carbohydrates as a ...
Sphaerotilus natans encrusted with nanoballshaped Fe(III) oxide
... G-179 (accession number AAC79443) (Fig. S2), respectively. The sequence comparison suggests that nitrate reductase from nitrate-dependent Fe(II) oxidizing bacteria could be quite different structurally from denitrifying bacteria, P. denitrificans and Pseudomonas sp. In this study, strain DSM 6575T a ...
... G-179 (accession number AAC79443) (Fig. S2), respectively. The sequence comparison suggests that nitrate reductase from nitrate-dependent Fe(II) oxidizing bacteria could be quite different structurally from denitrifying bacteria, P. denitrificans and Pseudomonas sp. In this study, strain DSM 6575T a ...
Chap 4. Growth and Metabolism
... Organic Compds + O2 --------------► CO2 + H2O + Energy + Mineral (Substrates, Energy source) ...
... Organic Compds + O2 --------------► CO2 + H2O + Energy + Mineral (Substrates, Energy source) ...
Carbon and energy distribution through propagation and fermentation
... Key aspects of redox balance 1. Typically, an oxidation is always coupled with a reduction. 2. If a preferred reduction pathway (glycerol) is compromised, cells will overproduce some other product (acetic acid, fusels) until cell is redox neutral (homeostasis). This slows growth in fermentation and ...
... Key aspects of redox balance 1. Typically, an oxidation is always coupled with a reduction. 2. If a preferred reduction pathway (glycerol) is compromised, cells will overproduce some other product (acetic acid, fusels) until cell is redox neutral (homeostasis). This slows growth in fermentation and ...
Thiorhodospira sibirica gen. nov., sp. nov., a new alkaliphilic purple
... Physiological properties ...
... Physiological properties ...
Chapter 2: Principles of Ecology - Seymour Community School District
... Biotic factors The living factors in an organism’s environment are called the biotic (by AH tihk) factors. Consider the biotic factors in the habitat of salmon shown in Figure 2.5. These biotic factors include all of the organisms that live in the water, such as other fish, algae, frogs, and microsc ...
... Biotic factors The living factors in an organism’s environment are called the biotic (by AH tihk) factors. Consider the biotic factors in the habitat of salmon shown in Figure 2.5. These biotic factors include all of the organisms that live in the water, such as other fish, algae, frogs, and microsc ...
Biochemistry I, Spring Term 2001 - Third Exam:
... C2. (15 pts) Answer ONE of the following three questions. i) In biosynthetic and degradative pathways, several steps are similar, often catalyzed by the same enzyme. Other steps are different, catalyzed by one or more different enzymes. As an example of the latter, pick one such step in either glyco ...
... C2. (15 pts) Answer ONE of the following three questions. i) In biosynthetic and degradative pathways, several steps are similar, often catalyzed by the same enzyme. Other steps are different, catalyzed by one or more different enzymes. As an example of the latter, pick one such step in either glyco ...
Chapter 2: Principles of Ecology - Bellbrook
... Biotic factors The living factors in an organism’s environment are called the biotic (by AH tihk) factors. Consider the biotic factors in the habitat of salmon shown in Figure 2.5. These biotic factors include all of the organisms that live in the water, such as other fish, algae, frogs, and microsc ...
... Biotic factors The living factors in an organism’s environment are called the biotic (by AH tihk) factors. Consider the biotic factors in the habitat of salmon shown in Figure 2.5. These biotic factors include all of the organisms that live in the water, such as other fish, algae, frogs, and microsc ...
No Slide Title
... 1. By flux of nitrogen through cycle - depends on diet lots protein in diet = carbon skeletons used for fuel, lots of urea starvation = breakdown muscle protein for energy, lots of urea All enzymes (CPS-I and 4 in cycle) synthesized at higher rates in starving animals and animals on high protein die ...
... 1. By flux of nitrogen through cycle - depends on diet lots protein in diet = carbon skeletons used for fuel, lots of urea starvation = breakdown muscle protein for energy, lots of urea All enzymes (CPS-I and 4 in cycle) synthesized at higher rates in starving animals and animals on high protein die ...
Microbial metabolism
Microbial metabolism is the means by which a microbe obtains the energy and nutrients (e.g. carbon) it needs to live and reproduce. Microbes use many different types of metabolic strategies and species can often be differentiated from each other based on metabolic characteristics. The specific metabolic properties of a microbe are the major factors in determining that microbe’s ecological niche, and often allow for that microbe to be useful in industrial processes or responsible for biogeochemical cycles.== Types of microbial metabolism ==All microbial metabolisms can be arranged according to three principles:1. How the organism obtains carbon for synthesising cell mass: autotrophic – carbon is obtained from carbon dioxide (CO2) heterotrophic – carbon is obtained from organic compounds mixotrophic – carbon is obtained from both organic compounds and by fixing carbon dioxide2. How the organism obtains reducing equivalents used either in energy conservation or in biosynthetic reactions: lithotrophic – reducing equivalents are obtained from inorganic compounds organotrophic – reducing equivalents are obtained from organic compounds3. How the organism obtains energy for living and growing: chemotrophic – energy is obtained from external chemical compounds phototrophic – energy is obtained from lightIn practice, these terms are almost freely combined. Typical examples are as follows: chemolithoautotrophs obtain energy from the oxidation of inorganic compounds and carbon from the fixation of carbon dioxide. Examples: Nitrifying bacteria, Sulfur-oxidizing bacteria, Iron-oxidizing bacteria, Knallgas-bacteria photolithoautotrophs obtain energy from light and carbon from the fixation of carbon dioxide, using reducing equivalents from inorganic compounds. Examples: Cyanobacteria (water (H2O) as reducing equivalent donor), Chlorobiaceae, Chromatiaceae (hydrogen sulfide (H2S) as reducing equivalent donor), Chloroflexus (hydrogen (H2) as reducing equivalent donor) chemolithoheterotrophs obtain energy from the oxidation of inorganic compounds, but cannot fix carbon dioxide (CO2). Examples: some Thiobacilus, some Beggiatoa, some Nitrobacter spp., Wolinella (with H2 as reducing equivalent donor), some Knallgas-bacteria, some sulfate-reducing bacteria chemoorganoheterotrophs obtain energy, carbon, and reducing equivalents for biosynthetic reactions from organic compounds. Examples: most bacteria, e. g. Escherichia coli, Bacillus spp., Actinobacteria photoorganoheterotrophs obtain energy from light, carbon and reducing equivalents for biosynthetic reactions from organic compounds. Some species are strictly heterotrophic, many others can also fix carbon dioxide and are mixotrophic. Examples: Rhodobacter, Rhodopseudomonas, Rhodospirillum, Rhodomicrobium, Rhodocyclus, Heliobacterium, Chloroflexus (alternatively to photolithoautotrophy with hydrogen)