Nuclear Chemistry PowerPoint
... • In 1902, Frederick Soddy proposed the theory that "radioactivity is the result of a natural change of an isotope of one element into an isotope of a different element." • Nuclear reactions involve changes in particles in an atom's nucleus and thus cause a change in the atom itself. All elements he ...
... • In 1902, Frederick Soddy proposed the theory that "radioactivity is the result of a natural change of an isotope of one element into an isotope of a different element." • Nuclear reactions involve changes in particles in an atom's nucleus and thus cause a change in the atom itself. All elements he ...
PS 2.2
... the weighted average of the masses of the naturally occurring isotopes of an element. The atomic mass of an element can be found on the periodic table. Since it is an average, it is usually not a whole number. ...
... the weighted average of the masses of the naturally occurring isotopes of an element. The atomic mass of an element can be found on the periodic table. Since it is an average, it is usually not a whole number. ...
MrsB-Chemistry
... B. A scientist thought that matter could not be divided into smaller pieces because chemical reactions only combine elements. They don’t cause elements to change into other elements. C. Alpha particles were used like bullets, and small, positively charged particles shot out from the center of the at ...
... B. A scientist thought that matter could not be divided into smaller pieces because chemical reactions only combine elements. They don’t cause elements to change into other elements. C. Alpha particles were used like bullets, and small, positively charged particles shot out from the center of the at ...
Section 1 Review
... 5. Infer Sodium and potassium are in the same group on the periodic table. Name ...
... 5. Infer Sodium and potassium are in the same group on the periodic table. Name ...
Lesson 13 - Highline Public Schools
... weighted average of the masses of the isotopes in a sample of the element. The most common isotope of an element, frequently has a mass that is close to the average atomic mass given in the periodic table. ...
... weighted average of the masses of the isotopes in a sample of the element. The most common isotope of an element, frequently has a mass that is close to the average atomic mass given in the periodic table. ...
2 - My George School
... Atoms with the same number of ________ but different numbers of ___________ Questions: What is the charge on an isotope? Will the mass number of the isotope be different than that of the more naturally abundant atom? ...
... Atoms with the same number of ________ but different numbers of ___________ Questions: What is the charge on an isotope? Will the mass number of the isotope be different than that of the more naturally abundant atom? ...
Dating the Earth Power Point
... radioactive date, the object being dated must contain a radioactive element such as uranium-235 or carbon 14. These elements are decaying by emitting a small part of the atom. They also become something new, uranium turns to lead, carbon 14 turns to carbon 12. By determining the ratio of uranium to ...
... radioactive date, the object being dated must contain a radioactive element such as uranium-235 or carbon 14. These elements are decaying by emitting a small part of the atom. They also become something new, uranium turns to lead, carbon 14 turns to carbon 12. By determining the ratio of uranium to ...
Isotopes, Ions Worksheet
... Different atoms of the same element have the SAME half-life. b) Do different isotopes have different half-lifes (t ½ )? YES Different isotopes have a different neutron number which results in different half-life 21. List THREE Nuclear Applications 1. _______________________ 2. ______________________ ...
... Different atoms of the same element have the SAME half-life. b) Do different isotopes have different half-lifes (t ½ )? YES Different isotopes have a different neutron number which results in different half-life 21. List THREE Nuclear Applications 1. _______________________ 2. ______________________ ...
Vocabulary for Periodic Table
... 3) Nucleus: the central region of an atom where most of the atom’s mass is found in protons and neutrons. 4) Electron: a negatively charged particle located outside an atom’s nucleus. 5) Atomic number: the number of protons in the nucleus of an atom. 6) Atomic mass number: the total number of proton ...
... 3) Nucleus: the central region of an atom where most of the atom’s mass is found in protons and neutrons. 4) Electron: a negatively charged particle located outside an atom’s nucleus. 5) Atomic number: the number of protons in the nucleus of an atom. 6) Atomic mass number: the total number of proton ...
Radioactive Isotopes and Nuclear Equations
... e , is emitted when a proton inside an atom decays to ...
... e , is emitted when a proton inside an atom decays to ...
Periodic Table Vocabulary Periodic Table – a chart that organizes
... Inert – elements and/or compounds that when put together are unable to react chemically. The Law of Conservation of Matter – a scientific law that states that during a chemical reaction, matter cannot be created or destroyed but can be changed into a different form. Period law- The chemical properti ...
... Inert – elements and/or compounds that when put together are unable to react chemically. The Law of Conservation of Matter – a scientific law that states that during a chemical reaction, matter cannot be created or destroyed but can be changed into a different form. Period law- The chemical properti ...
atomic structure - IGCSE STUDY BANK
... Isotopes are atoms of the same element with different numbers of neutrons. This gives each isotope of the element a different mass or nucleon number but being the same element they have the same atomic or proton number. There are small physical differences between the isotopes eg the heavier isotope ...
... Isotopes are atoms of the same element with different numbers of neutrons. This gives each isotope of the element a different mass or nucleon number but being the same element they have the same atomic or proton number. There are small physical differences between the isotopes eg the heavier isotope ...
Unit IV Review Guide: Atomic Structure and Nuclear Reactions
... 3. Which subatomic particle was discovered by researchers working with cathode-ray tubes? 4. Briefly explain how Rutherford discovered the nucleus? Draw a diagram to help explain. ...
... 3. Which subatomic particle was discovered by researchers working with cathode-ray tubes? 4. Briefly explain how Rutherford discovered the nucleus? Draw a diagram to help explain. ...
Structure of the Atom
... 4. What structural characteristics do all hydrogen atoms have in common? ...
... 4. What structural characteristics do all hydrogen atoms have in common? ...
Atomic Structure - hrsbstaff.ednet.ns.ca
... So, what’s up with all these isotopes anyway? In nature elements are not made up of atoms that are all exactly the same! Some will be heavier than others, even though they are still the same type of atom. C-12 and C-14 are both Carbon, with all the usual Carbon properties, but the C-14 has two more ...
... So, what’s up with all these isotopes anyway? In nature elements are not made up of atoms that are all exactly the same! Some will be heavier than others, even though they are still the same type of atom. C-12 and C-14 are both Carbon, with all the usual Carbon properties, but the C-14 has two more ...
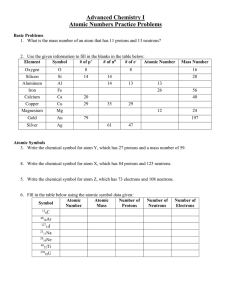
Atomic Numbers Practice Problems
... 7. With respect to the three fundamental particles of atoms, which numbers of particles are the same between two isotopes, and which ones are different? ...
... 7. With respect to the three fundamental particles of atoms, which numbers of particles are the same between two isotopes, and which ones are different? ...
Name Period Nuclear Study Packet Set 1 1. What subatomic
... sample contains 34.2 mg of K-42. How much did it contain yesterday at the same time. 4. What percent of a sample of a radioactive element whose half life is 5 years will decay after 25 years? 5. What are some ways that nuclear reactions are being used today? Do not list any ways that we have tal ...
... sample contains 34.2 mg of K-42. How much did it contain yesterday at the same time. 4. What percent of a sample of a radioactive element whose half life is 5 years will decay after 25 years? 5. What are some ways that nuclear reactions are being used today? Do not list any ways that we have tal ...
Chemistry Review: Antoine Lavoisier (1743
... the same number of electrons, their chemical properties will be exactly the same. However, they have different masses due to the different number of neutrons; therefore, they will have slightly different properties that relate to mass. (Ex. Density , melting and boiling point). Also. Since the neutr ...
... the same number of electrons, their chemical properties will be exactly the same. However, they have different masses due to the different number of neutrons; therefore, they will have slightly different properties that relate to mass. (Ex. Density , melting and boiling point). Also. Since the neutr ...
Atomic Structure
... •Even though isotopes have different amounts of neutrons they are still chemically alike since they have the same number of protons and electrons. •To find the most common isotope round the atomic mass to nearest whole number. -Ex: Carbon-12 is the most common isotope of carbon Which isotope is the ...
... •Even though isotopes have different amounts of neutrons they are still chemically alike since they have the same number of protons and electrons. •To find the most common isotope round the atomic mass to nearest whole number. -Ex: Carbon-12 is the most common isotope of carbon Which isotope is the ...
VOCABULARY name, date, hour: Fill in the number of each term
... ___ substance that is a mixture of two or more metals ___ columns of the periodic table; also known as groups ___ number of protons carried by the nucleus of an atom ___ element with an imbalance in the number of neutrons and protons ___ uncharged particle found in the nucleus of an atom ___ physica ...
... ___ substance that is a mixture of two or more metals ___ columns of the periodic table; also known as groups ___ number of protons carried by the nucleus of an atom ___ element with an imbalance in the number of neutrons and protons ___ uncharged particle found in the nucleus of an atom ___ physica ...
C2- Topic 1: Atomic structure and the periodic table. Assessable
... - arranged the elements, known at that time, in a periodic table by using properties of these elements and their compounds - used his table to predict the existence and properties of some elements not then discovered ...
... - arranged the elements, known at that time, in a periodic table by using properties of these elements and their compounds - used his table to predict the existence and properties of some elements not then discovered ...
Beryllium isotopes in geochronology Cosmogenic Be and Be
... gamma rays (gamma radiation) – a stream of high-energy electromagnetic radiation given off by an atomic nucleus undergoing radioactive decay. The energies of gamma rays are higher than those of X-rays; thus, gamma rays have greater penetrating power. half-life (radioactive) – the time interval that ...
... gamma rays (gamma radiation) – a stream of high-energy electromagnetic radiation given off by an atomic nucleus undergoing radioactive decay. The energies of gamma rays are higher than those of X-rays; thus, gamma rays have greater penetrating power. half-life (radioactive) – the time interval that ...
Chapter 4 Study Guide Physical Science 1. The word atom comes
... 2. Halogens are very reactive elements located in Group _______of the periodic table. 3. The nucleus of an atom has a(n) ____________________ electric charge. 4. Carbon is found in group ______ of the periodic table. 5. Bohr’s model of the atom compares electrons to ____________________. 6. Elements ...
... 2. Halogens are very reactive elements located in Group _______of the periodic table. 3. The nucleus of an atom has a(n) ____________________ electric charge. 4. Carbon is found in group ______ of the periodic table. 5. Bohr’s model of the atom compares electrons to ____________________. 6. Elements ...