ChLM Final Review Name: Period: Base Knowledge 1. Classify the
... 1. Classify the following as observations or inferences a) The liquid is green because food coloring was added. b) The beaker has green liquid in it. c) The beaker can hold up to 250 mL. d) The beaker will be the best tool for this lab. 2. Measure the following, circle your estimated digit and inclu ...
... 1. Classify the following as observations or inferences a) The liquid is green because food coloring was added. b) The beaker has green liquid in it. c) The beaker can hold up to 250 mL. d) The beaker will be the best tool for this lab. 2. Measure the following, circle your estimated digit and inclu ...
Atoms: The Building Blocks of Matter
... lost or gained with oxidation numbers (also known as charges) Ions are charged particles –when an atom has too many or too few electrons to be neutral No change to the nucleus Proton and neutrons stay the same number. ...
... lost or gained with oxidation numbers (also known as charges) Ions are charged particles –when an atom has too many or too few electrons to be neutral No change to the nucleus Proton and neutrons stay the same number. ...
Lecture 2
... •Atoms are indestructible and retain their identity in all chemical reactions. •All of the atoms of a given chemical element are identical in mass and in all other properties. •Different elements have different kinds of atoms; these atoms differ in mass from element to element. •Compounds consist of ...
... •Atoms are indestructible and retain their identity in all chemical reactions. •All of the atoms of a given chemical element are identical in mass and in all other properties. •Different elements have different kinds of atoms; these atoms differ in mass from element to element. •Compounds consist of ...
File - Norris Science
... the tiny alpha particles would pass through the gold atoms and fly straight into the screen. ...
... the tiny alpha particles would pass through the gold atoms and fly straight into the screen. ...
2.2 Periodic Trends
... What are the trends that occur in the periodic table by organizing elements by their atomic number? Periodic trends are specific patterns that are present in the periodic table that illustrate different aspects of a certain element. Periodic trends, arising from the arrangement of the periodic t ...
... What are the trends that occur in the periodic table by organizing elements by their atomic number? Periodic trends are specific patterns that are present in the periodic table that illustrate different aspects of a certain element. Periodic trends, arising from the arrangement of the periodic t ...
ppt
... but the actual atomic mass is not exactly the sum of the masses of the nucleons (p + n). ● The electrons do have a small mass. ● Some mass is gained/lost in the energy binding ...
... but the actual atomic mass is not exactly the sum of the masses of the nucleons (p + n). ● The electrons do have a small mass. ● Some mass is gained/lost in the energy binding ...
Understanding the Atom GN
... When atoms of the same element have different numbers of neutrons they are called ____________________. Isotope – ________________________________________________________________________ Most elements have ______________________ isotopes. Mass Number - ________________________________________ ...
... When atoms of the same element have different numbers of neutrons they are called ____________________. Isotope – ________________________________________________________________________ Most elements have ______________________ isotopes. Mass Number - ________________________________________ ...
Radioactive Isotopes and Nuclear Equations
... b. Identify the radioactive isotope that decays to produce a neutron and phosphorus-30 when bombarded with an alpha particle. ...
... b. Identify the radioactive isotope that decays to produce a neutron and phosphorus-30 when bombarded with an alpha particle. ...
BellWork 2/16/2015
... element has a different atomic mass than another isotope of the same element ◦ Remember: C-12 has an atomic mass of 12 and C14 has an atomic mass of 14! ...
... element has a different atomic mass than another isotope of the same element ◦ Remember: C-12 has an atomic mass of 12 and C14 has an atomic mass of 14! ...
+ mass isotope 2
... Neutrons should be blank or have an N. • In a circle around the nucleus are the electrons. Electrons should have a minus sign or an e. ...
... Neutrons should be blank or have an N. • In a circle around the nucleus are the electrons. Electrons should have a minus sign or an e. ...
The Nuclear Atom
... • the atomic mass on periodic table is the WEIGHTED AVERAGE MASS of “all” the isotopes of that element – this is based on an isotope’s natural abundance • the percentage of each isotope of an element that occurs in nature ...
... • the atomic mass on periodic table is the WEIGHTED AVERAGE MASS of “all” the isotopes of that element – this is based on an isotope’s natural abundance • the percentage of each isotope of an element that occurs in nature ...
File
... Most common isotope method (mci). Round the average atomic mass value from the periodic table to the nearest whole number Carbon’s Atomic mass is 12.011 = round to 12 Manganese’s Atomic mass is 54.95 = round to 55 ...
... Most common isotope method (mci). Round the average atomic mass value from the periodic table to the nearest whole number Carbon’s Atomic mass is 12.011 = round to 12 Manganese’s Atomic mass is 54.95 = round to 55 ...
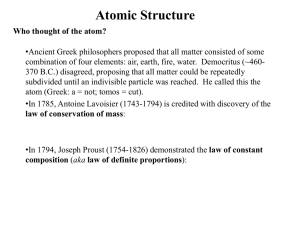
What is the history of chemistry and elements
... 2. What is the structure of an atom? 3. How are ions formed from atoms? History 2400 year ago Greek philosophers proposed that everything was made of four basic substances – air, water, fire, and earth. Today chemists know that there are 100+ basic substances, or elements. Everything on Earth ...
... 2. What is the structure of an atom? 3. How are ions formed from atoms? History 2400 year ago Greek philosophers proposed that everything was made of four basic substances – air, water, fire, and earth. Today chemists know that there are 100+ basic substances, or elements. Everything on Earth ...
Chapter 18
... • Dimitri Mendeleevarranged all the elements known in order of increasing atomic masses and discovered a pattern • Today’s Periodic Table— elements are arranged by increasing atomic number and by changes in physical and chemical properties ...
... • Dimitri Mendeleevarranged all the elements known in order of increasing atomic masses and discovered a pattern • Today’s Periodic Table— elements are arranged by increasing atomic number and by changes in physical and chemical properties ...
Vocabulary and Section Summary
... Name ______________________________ Class___________________Date__________________ ...
... Name ______________________________ Class___________________Date__________________ ...
Atoms - ChemistryatBiotech
... lost or gained with oxidation numbers (also known as charges) Ions are charged particles –when an atom has too many or too few electrons to be neutral No change to the nucleus Proton and neutrons stay the same number. ...
... lost or gained with oxidation numbers (also known as charges) Ions are charged particles –when an atom has too many or too few electrons to be neutral No change to the nucleus Proton and neutrons stay the same number. ...
14 more about the atomic number
... More about the atomic number Each element in the Periodic Table has its own atomic number. The atomic number is important it gives the number of protons in an atom of an element. Since atoms are neutral and the charge on an electron is equal and opposite to the charge on a proton, the atomic number ...
... More about the atomic number Each element in the Periodic Table has its own atomic number. The atomic number is important it gives the number of protons in an atom of an element. Since atoms are neutral and the charge on an electron is equal and opposite to the charge on a proton, the atomic number ...
Atomic Structure
... a proton, but unlike the proton, has no charge. k. Atoms of the same element that have a different number of neutrons (hence …different mass numbers) l. This part of the atom houses protons and neutrons. ...
... a proton, but unlike the proton, has no charge. k. Atoms of the same element that have a different number of neutrons (hence …different mass numbers) l. This part of the atom houses protons and neutrons. ...
PP - myndrs.com
... be made or destroyed All atoms of the same element are identical Different elements have different types of atoms Chemical reactions occur when atoms are rearranged Compounds are formed from atoms of the different elements coming together. ...
... be made or destroyed All atoms of the same element are identical Different elements have different types of atoms Chemical reactions occur when atoms are rearranged Compounds are formed from atoms of the different elements coming together. ...
Structure of an Atom structure_of_atom
... be made or destroyed All atoms of the same element are identical Different elements have different types of atoms Chemical reactions occur when atoms are rearranged Compounds are formed from atoms of the different elements coming together. ...
... be made or destroyed All atoms of the same element are identical Different elements have different types of atoms Chemical reactions occur when atoms are rearranged Compounds are formed from atoms of the different elements coming together. ...
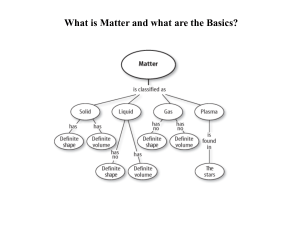
Matter and the Periodic Table
... Groups: Also known as families, the 18 vertical rows The elements in each group have similar characteristics. ...
... Groups: Also known as families, the 18 vertical rows The elements in each group have similar characteristics. ...
Radioactive Isotopes and Nuclear Equations
... b. Identify the radioactive isotope that decays to produce a neutron and phosphorus-30 when bombarded with an alpha particle. ...
... b. Identify the radioactive isotope that decays to produce a neutron and phosphorus-30 when bombarded with an alpha particle. ...
Quiz review
... What family of elements has only 1 valence electron? Which family of elements has a filled valence shell? Which is larger? A Lithium atom or a Lithium ion? Which is larger? A Chlorine atom or a Chlorine ion? What is the next larger atom than sodium in the same family? How many atoms are larger than ...
... What family of elements has only 1 valence electron? Which family of elements has a filled valence shell? Which is larger? A Lithium atom or a Lithium ion? Which is larger? A Chlorine atom or a Chlorine ion? What is the next larger atom than sodium in the same family? How many atoms are larger than ...