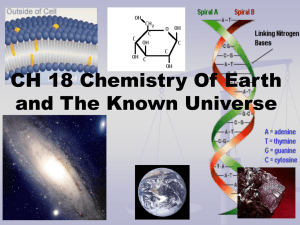
Chapter 4 Study Guide Physical Science 1. The word atom comes
... 2. Halogens are very reactive elements located in Group _______of the periodic table. 3. The nucleus of an atom has a(n) ____________________ electric charge. 4. Carbon is found in group ______ of the periodic table. 5. Bohr’s model of the atom compares electrons to ____________________. 6. Elements ...
... 2. Halogens are very reactive elements located in Group _______of the periodic table. 3. The nucleus of an atom has a(n) ____________________ electric charge. 4. Carbon is found in group ______ of the periodic table. 5. Bohr’s model of the atom compares electrons to ____________________. 6. Elements ...
Chapter 18 Notes
... Dimitri Mendeleev (18341907) organized information about all the known elements in a table that visually organized the similarities between them. Mendeleev placed each element on the table in a certain row and column based on its properties. ...
... Dimitri Mendeleev (18341907) organized information about all the known elements in a table that visually organized the similarities between them. Mendeleev placed each element on the table in a certain row and column based on its properties. ...
The study of biology can help you better understand human
... Which subatomic particle determines chemical properties of an element? ...
... Which subatomic particle determines chemical properties of an element? ...
L.O.
... I have some understanding but I need to revise this some more I don’t know this or I need help because I don’t understand it ...
... I have some understanding but I need to revise this some more I don’t know this or I need help because I don’t understand it ...
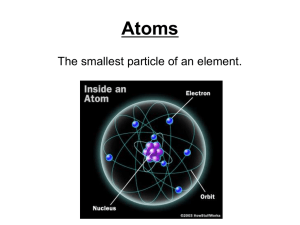
Classifying Atoms
... appears on pages 698–699 of the Appendix. Of the more than 100 known elements listed there, 92 occur naturally on Earth in significant amounts. The rest are synthetic elements produced by scientists. In each row of the periodic table, elements are listed from left to right in order of increasing num ...
... appears on pages 698–699 of the Appendix. Of the more than 100 known elements listed there, 92 occur naturally on Earth in significant amounts. The rest are synthetic elements produced by scientists. In each row of the periodic table, elements are listed from left to right in order of increasing num ...
Stable isotope Relative atomic mass Mole fraction Os 183.952 489
... gamma rays (gamma radiation) – a stream of high-energy electromagnetic radiation given off by an atomic nucleus undergoing radioactive decay. The energies of gamma rays are higher than those of X-rays; thus, gamma rays have greater penetrating power. half-life (radioactive) – the time interval that ...
... gamma rays (gamma radiation) – a stream of high-energy electromagnetic radiation given off by an atomic nucleus undergoing radioactive decay. The energies of gamma rays are higher than those of X-rays; thus, gamma rays have greater penetrating power. half-life (radioactive) – the time interval that ...
Lecture4
... Alpha particles (named after and denoted by the first letter in the Greek alphabet, α) consist of two protons and two neutrons bound together into a particle identical to a helium nucleus, which is produced in the process of alpha decay. The alpha particle can be written as He2+, 42He2+ or 42He .So ...
... Alpha particles (named after and denoted by the first letter in the Greek alphabet, α) consist of two protons and two neutrons bound together into a particle identical to a helium nucleus, which is produced in the process of alpha decay. The alpha particle can be written as He2+, 42He2+ or 42He .So ...
Honors Review Unit 2 answers
... Proposed the “Plum Pudding” model of the atom. _____Thomson________ Used the word “atomos” to describe matter. _____Democritos________ Called the “Father of Modern Chemistry”. ___Lavoisier_________ Discovered the neutron. _____Chadwick___________ Created the first atomic theory based on experimental ...
... Proposed the “Plum Pudding” model of the atom. _____Thomson________ Used the word “atomos” to describe matter. _____Democritos________ Called the “Father of Modern Chemistry”. ___Lavoisier_________ Discovered the neutron. _____Chadwick___________ Created the first atomic theory based on experimental ...
Atomic Structure and the Periodic Table Vocabulary
... Choose the vocabulary word that matches each description by circling it. Use the bolded words in the sentences as clues. 19. Sometimes this is called a family of elements because these elements seem to be ...
... Choose the vocabulary word that matches each description by circling it. Use the bolded words in the sentences as clues. 19. Sometimes this is called a family of elements because these elements seem to be ...
IPC Atoms and Periodic Table
... start with actinium (Ac) at atomic number 89 and finishing up with lawrencium (Lr) at number 103. • They are all radioactive and some are not found in nature. ...
... start with actinium (Ac) at atomic number 89 and finishing up with lawrencium (Lr) at number 103. • They are all radioactive and some are not found in nature. ...
Chapter 6 Review“The Periodic Table”
... Review“The Periodic Table” 1. How is the number of neutrons in the nucleus of an atom calculated? 2. All atoms are neutral, with the number of protons equaling the ___. 3. Isotopes of the same element have different _____. 4. Using the periodic table, determine the number of neutrons in 16O. 5. What ...
... Review“The Periodic Table” 1. How is the number of neutrons in the nucleus of an atom calculated? 2. All atoms are neutral, with the number of protons equaling the ___. 3. Isotopes of the same element have different _____. 4. Using the periodic table, determine the number of neutrons in 16O. 5. What ...
Atomic Structure AKS Correlation Use the modern atomic theory to
... Discovered e-, experiment, predictable shells, indivisible All matter made of plum pudding discovered p+, Bohr model 1st to think atoms model Atoms mostly about matter Matter cannot be empty space, dense and its’ makecreated or center up of atoms destroyed Atoms of same element look the same Chem re ...
... Discovered e-, experiment, predictable shells, indivisible All matter made of plum pudding discovered p+, Bohr model 1st to think atoms model Atoms mostly about matter Matter cannot be empty space, dense and its’ makecreated or center up of atoms destroyed Atoms of same element look the same Chem re ...
1.2 Atomic Theory
... The average atomic mass for magnesium found on the periodic table is a weighted average of the three isotopes: 24.31 g of Mg Radioactivity: spontaneous decay of nuclei, releasing energy and subatomic particles Radioisotopes: an unstable isotope of an element, which undergoes radioactive decay ...
... The average atomic mass for magnesium found on the periodic table is a weighted average of the three isotopes: 24.31 g of Mg Radioactivity: spontaneous decay of nuclei, releasing energy and subatomic particles Radioisotopes: an unstable isotope of an element, which undergoes radioactive decay ...
1st Term Review
... 13. What is the mass of grams of 0.500 moles of Au? 14. Based on the gold foil experiment, what did Rutherford conclude about the atom? 15. An atom of chromium-60 contains how many protons, neutron and electrons? 16. What is the difference between a compound and an element? 17. What is the electron ...
... 13. What is the mass of grams of 0.500 moles of Au? 14. Based on the gold foil experiment, what did Rutherford conclude about the atom? 15. An atom of chromium-60 contains how many protons, neutron and electrons? 16. What is the difference between a compound and an element? 17. What is the electron ...
Practice Test #2 - smhs
... for Ag as 107.868 amu. Find the percent abundance of the lighter isotope of Ag. 107.868 = 106.9041 (X) + 108.9047 (1.000 - X) -1.0367 = -2.0006 X X = 0.51819 ...
... for Ag as 107.868 amu. Find the percent abundance of the lighter isotope of Ag. 107.868 = 106.9041 (X) + 108.9047 (1.000 - X) -1.0367 = -2.0006 X X = 0.51819 ...
Practice Test #2 - smhs
... 15._____ His oil-drop experiment enabled scientists to measure the charge on the electron. 16._____ He concluded that the atom had a small, compact, positively-charged nucleus surrounded by electrons based on his gold-foil experiment. 17._____ He invented the mass spectrograph, an instrument that is ...
... 15._____ His oil-drop experiment enabled scientists to measure the charge on the electron. 16._____ He concluded that the atom had a small, compact, positively-charged nucleus surrounded by electrons based on his gold-foil experiment. 17._____ He invented the mass spectrograph, an instrument that is ...
Name Test Review Chapters 4 and 25 Honors Chemistry 1. Fill in
... Discovered the charged nucleus (small) is the center with 99.9% of the atom as empty space where the electrons reside. _____________________________ Used an “oil drop” experiment to discover the charge and mass of an electron. __________________ Proposed the “Plum Pudding” model of the atom. _______ ...
... Discovered the charged nucleus (small) is the center with 99.9% of the atom as empty space where the electrons reside. _____________________________ Used an “oil drop” experiment to discover the charge and mass of an electron. __________________ Proposed the “Plum Pudding” model of the atom. _______ ...
Name Test Review Chemistry Unit 2: The Atom 1. Fill in the blank
... Discovered the charged nucleus (small) is the center with 99.9% of the atom as empty space where the electrons reside. _____________________________ Used an “oil drop” experiment to discover the charge and mass of an electron. __________________ Proposed the “Plum Pudding” model of the atom. _______ ...
... Discovered the charged nucleus (small) is the center with 99.9% of the atom as empty space where the electrons reside. _____________________________ Used an “oil drop” experiment to discover the charge and mass of an electron. __________________ Proposed the “Plum Pudding” model of the atom. _______ ...
Quiz: The Atom (Open Notes)
... 10. T or F A neutron carries a positive charge. 11. T or F A proton has a charge that is equal in force but opposite in charge to each electron. 12. T or F Protons and electrons are about equal in mass. 13. T or F The mass of an atom depends on the number of protons and neutrons in its nucleus. 14. ...
... 10. T or F A neutron carries a positive charge. 11. T or F A proton has a charge that is equal in force but opposite in charge to each electron. 12. T or F Protons and electrons are about equal in mass. 13. T or F The mass of an atom depends on the number of protons and neutrons in its nucleus. 14. ...
File
... compound: a substance made up of atoms of two ore more different elements joined by chemical bonds. atomic number: the number of protons in the nucleus of an atom. (The atomic number is the same for all atoms of an element.) chemical symbol: a one, two, or three-letter abbreviation of the name of an ...
... compound: a substance made up of atoms of two ore more different elements joined by chemical bonds. atomic number: the number of protons in the nucleus of an atom. (The atomic number is the same for all atoms of an element.) chemical symbol: a one, two, or three-letter abbreviation of the name of an ...
Chap 7: Around the Room Review
... 1. The central part of an atom is called the _____ 2. A proton has a _____ charge. 3. The atomic number tells us __________. 4. Nitrogen’s atomic number is 7. An isotope of nitrogen containing 7 neutrons would be nitrogen_____. 5. How does the size of a negative ion compare to the size of the atom t ...
... 1. The central part of an atom is called the _____ 2. A proton has a _____ charge. 3. The atomic number tells us __________. 4. Nitrogen’s atomic number is 7. An isotope of nitrogen containing 7 neutrons would be nitrogen_____. 5. How does the size of a negative ion compare to the size of the atom t ...
Exam III Review
... c. The smallest particle of an element that retains the chemical identity of that element. d. An artificially assembled unit that contains protons and electrons. 14. J.J. Thomson concluded that a cathode ray contains negatively charged particles by studying how a. The cathode ray produced a green sp ...
... c. The smallest particle of an element that retains the chemical identity of that element. d. An artificially assembled unit that contains protons and electrons. 14. J.J. Thomson concluded that a cathode ray contains negatively charged particles by studying how a. The cathode ray produced a green sp ...