* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Dating the Earth Power Point
Survey
Document related concepts
Transcript
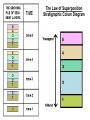
Dating the Earth Grade 8 Science The Principle of Uniformitarianism • The past history of the Earth must be explained by what we can see happening today. • Or: “the present is the key to the past” Two Methods • Relative Dating – list order of events, not ages – Laws and Principles of Stratigraphy • Absolute dating – actual age of events in years. – Radiometric dating Relative Dating Grade 8 Science Relative Dating • Is the science determining the relative order of past events, without necessarily determining their absolute age. • Identifying which rocks formed first, second, third and so on. • Tells us the sequence in which events occurred, not how long ago they occurred Laws and Principles of Stratigraphy • • • • • Law of Superposition Law of Original Horizontality Law of Cross Cutting Relationships Law of Lateral Continuity Law of Included Fragments Law of Superposition • In an unaltered sequence of sedimentary rocks each bed or layer is older than the one above it and younger than the one below it. The Law of Superposition Stratigraphic Colum Diagram Law of Original Horizontality • Sediment is generally deposited in a horizontal position • Flat layers are undisturbed • If rock layers are tilted or bent, then they must have been moved sometime after they were first deposited. • Law of cross-cutting Relationships a geologic feature which cuts through another is the younger of the two features. • Applies to faults or igneous intrusions (dikes, etc.) • Faults or intrusions are younger than the rocks affected Law of Lateral Continuity • Layers of sediment initially extend laterally in all directions result in rocks that are otherwise similar, but are now separated by a valley or other erosional feature, • They can be assumed to be originally continuous. The Law of Included Fragments • Fragments of rock that are contained (or included) within a host rock are older than the host rock. Absolute Dating by the Use of Radioactive Isotopes absolute dating the process of establishing the age of an object by determining the number of years it has existed Relative Dating http://evolution.berkeley.edu/evosite/lines/IIIAchronology.shtml Absolute Dating Absolute Dating • Does not mean it isn’t without error • Radiometric dating is the most common type of absolute dating. Carbon element box What can be determined? Element Carbon 6 Symbol Atomic Number C 12.01 Atomic Mass Atoms and Isotopes • If you change the # of neutrons, the element stays the same, but the mass changes • ISOTOPES - atoms of the same element that have different numbers of neutrons Carbon-14 is also referred to as: C-14 Carbon-14 • Types of carbon (isotopes) Atomic mass 9 14 16 6 6 6 Atomic number Atoms & Isotopes • Some are stable and some are unstable • When an isotope is unstable it will decay over time and turn into another atom • If an isotope is unstable it is considered to be radioactive • The process of breaking down is called radioactive decay. • Original isotope is the parent • New atom is the daughter The nucleus of an atom changes into a new element – The proton number (atomic number) must change – A neutron changes into a proton 14 6 14 7 Atoms and Isotopes • If you change the # of protons, the element changes & the mass changes. • Which element has 7 protons? Nitrogen • If I take away one proton, I would only have six! Which element has 6 protons? Carbon • If I add one proton to the original 7, I will have 8. Which element has 8 protons? Oxygen Radioactive Decay • Alpha decay - Alpha decay is caused when there are too many protons in a nucleus. In this case the element will emit radiation in the form of positively charged particles called alpha particles. • Beta decay - Beta decay is caused when there are too many neutrons in a nucleus. In this case the element will emit radiation in the form of negatively charged particles called beta particles. • Gamma decay - Gamma decay occurs when there is too much energy in the nucleus. In this case gamma particles with no overall charge are emitted from the element. Radioactive Decay • When an atom (or isotope) undergoes radioactive decay it can lost some atomic particles (protons, neutrons or electrons) and turn from one element into another. • Radioactive decay – the process by which an unstable element naturally changes into another element that is stable. Radioactive Elements Decay • The universe is full of naturally occurring radioactive elements. Radioactive atoms are inherently unstable; over time, radioactive “parent atoms” decay into stable “daughter atoms.” • Most commonly occurs in igneous rock. • When molten rock cools radioactive atoms are trapped inside. • Afterward they decay. How Long Does Radioactive Decay Take? • Half-Life - the time it takes for half of the atoms (radioactive isotopes) in a sample to decay. – Measured in seconds, minutes, years, etc. – Each isotope has its own unique half-life. • From thousandths of a second to billions of years For example: The half life of carbon-14 is 5,730 years. If you have a sample of carbon-14 with 1,000 atoms how many atoms will be left after one half-life? 500 Common Isotope Pairs Half-Life • After two half-lives how much of the original isotope will remain? One-fourth • After three half-lives how much of the original isotope will remain? One-eighth . If isotope X has a half life of 20 years and it decays over a period of 100 years. How many half lives did it go through? For example: • An isotope of cesium (cesium -137) has a halflife of 30 years. If 1 gram of cesium – 137 disintegrates over a period of 90 years, how many grams of cesium-137 would remain? Time (yrs.) Mass (g) For example: • An isotope of argon has a half-life of 500 years. If 100 grams of argon disintegrates over a period of 2000 years, how many grams of argon would remain? Time (yrs.) Mass (g) Twizzler activity Background • Finding out how old something is helps scientists understand the history of Earth and determine evolutionary pathways. Radioactive dating is an important tool scientists use to do this. To find a radioactive date, the object being dated must contain a radioactive element such as uranium-235 or carbon 14. These elements are decaying by emitting a small part of the atom. They also become something new, uranium turns to lead, carbon 14 turns to carbon 12. By determining the ratio of uranium to lead or carbon 14 to carbon 12, it can be determined how long that item has been around. In this activity you will model the decay of three different “radioactive” atoms, each with a different half-life. Half-life is a common way to describe the length of time it takes for half the atoms in a particular element to decay. Purpose • Purpose: The purpose of this lab is to use licorice as a model of radioactive atomic decay. You will cut several pieces of licorice in half to model the decay of different “elements.” This should help you understand the concept of a half-life and how it is used in dating fossils as evidence for evolution. Procedure 1. Obtain a piece of each color licorice from your teacher. Different colors of licorice represent different elements in nature. For example the red licorice could represent an element like carbon 14. 2. The color of the licorice determines its half-life. Each time a half-life passes 1/2 of the element decays. 3. In each table record the initial amount of each element. Do this by massing the piece of licorice. 4. Cut your licorice in half. Find the mass of the piece you cut. This is the amount (in grams) of the element the licorice “decayed” into. Record in your data table. 5. Measure the amount of licorice you have left. Record in your data table. The time it took for this decay is the half-life. 6. Continue this process until you cannot cut your licorice any more (or you cannot determine the mass using the electronic balance). 7. Label your graph x-axis “years” and y-axis “amount in grams to start”. Try to fill up as much of the graph paper as you can. 8. Graph your data. (Use a different color for each element.) 9. Repeat steps 1-8 for each piece of licorice. Color (atom) Red-1 Black-1 Half-Life (years) 2000 500 Radiometric Ages Atoms Don’t Age the Way We Do 1 2 3 4 Start with 16 baby aliens Have 70 year half-lives 4 half-lives = 280 years Each atom has a 50% chance of decaying during a half-life. http://www.colorado.edu/physics/2000/isotopes/ima ges/age280_baby.jpg