Lecture notes 2: Quantum mechanics in a nutshell
... can be built up by neglecting electron spin (but do keep the exclusion principle). This means any electron configuration can have two electrons — one with spin up, the other with spin down. Assume further that each electron moves in a central field which is the combined field of the nucleus and all ...
... can be built up by neglecting electron spin (but do keep the exclusion principle). This means any electron configuration can have two electrons — one with spin up, the other with spin down. Assume further that each electron moves in a central field which is the combined field of the nucleus and all ...
Document
... • Quantum physics explains the energy levels of atoms with enormous accuracy. This is possible, since these levels have long lifetime (uncertainty relation for E, t). • Radiation from atoms and molecules enables the most accurate time and length measurements: Atomic clocks • Quantum physics explai ...
... • Quantum physics explains the energy levels of atoms with enormous accuracy. This is possible, since these levels have long lifetime (uncertainty relation for E, t). • Radiation from atoms and molecules enables the most accurate time and length measurements: Atomic clocks • Quantum physics explai ...
• Quantum physics explains the energy levels of atoms with
... • Quantum physics explains the energy levels of atoms with enormous accuracy. This is possible, since these levels have long lifetime (uncertainty relation for E, t). • Radiation from atoms and molecules enables the most accurate time and length measurements: Atomic clocks • Quantum physics explai ...
... • Quantum physics explains the energy levels of atoms with enormous accuracy. This is possible, since these levels have long lifetime (uncertainty relation for E, t). • Radiation from atoms and molecules enables the most accurate time and length measurements: Atomic clocks • Quantum physics explai ...
Chapter 4 Study Guide-Atomic Structure Define the following terms
... What is the mass of a proton and a neutron? 1 amu each Draw a picture of Rutherford’s Atomic Model. Neutrons and protons in center (nucleus) with electrons moving around the nucleus. A sample of Zirconium has a mass of 91.22g and 6.02x1023 atoms. How much mass does each atom have? (SHOW YOUR WORK) 9 ...
... What is the mass of a proton and a neutron? 1 amu each Draw a picture of Rutherford’s Atomic Model. Neutrons and protons in center (nucleus) with electrons moving around the nucleus. A sample of Zirconium has a mass of 91.22g and 6.02x1023 atoms. How much mass does each atom have? (SHOW YOUR WORK) 9 ...
Unit 1 - Learning Objectives
... Cars with catalytic converters only use ‘lead-free’ petrol to prevent poisoning of the catalyst. Enzymes catalyse the chemical reactions which take place in the living cells of plants and animals. There are many everyday examples of uses of enzymes. Enzymes are used in many industrial proces ...
... Cars with catalytic converters only use ‘lead-free’ petrol to prevent poisoning of the catalyst. Enzymes catalyse the chemical reactions which take place in the living cells of plants and animals. There are many everyday examples of uses of enzymes. Enzymes are used in many industrial proces ...
Chemistry 199 - Oregon State chemistry
... Electrical current is produced with a fuel cell utilizing the above process (see Fuel Cell handout from Wedneday, May 8). ...
... Electrical current is produced with a fuel cell utilizing the above process (see Fuel Cell handout from Wedneday, May 8). ...
Random Laser - Department of Physics
... semiconductor is fully and uniquely characterized by its band structure. At zero temperature, electrons occupy only the lower energy bands, and the highest such occupied band is called valence band. The next highest energy band is termed the conduction band. The two most important and useful quanti ...
... semiconductor is fully and uniquely characterized by its band structure. At zero temperature, electrons occupy only the lower energy bands, and the highest such occupied band is called valence band. The next highest energy band is termed the conduction band. The two most important and useful quanti ...
File
... 6. The effective nuclear charge experienced by the outermost electron of Na is different than the effective nuclear charge experienced by the outermost electron of Ne. This difference best accounts for which of the following? A. Na has a greater density at standard conditions than Ne. B. Na has a lo ...
... 6. The effective nuclear charge experienced by the outermost electron of Na is different than the effective nuclear charge experienced by the outermost electron of Ne. This difference best accounts for which of the following? A. Na has a greater density at standard conditions than Ne. B. Na has a lo ...
Graphene Graphene is allotrope of carbon
... Graphene is one of the strongest,lightest and most conductive materialsa known to human-kind.It is highly transport. Graphene’s properties are attractive to materials .Scientists and electrical engineers made it possible to build circuits that are smaller and faster than silicon. It is applied in va ...
... Graphene is one of the strongest,lightest and most conductive materialsa known to human-kind.It is highly transport. Graphene’s properties are attractive to materials .Scientists and electrical engineers made it possible to build circuits that are smaller and faster than silicon. It is applied in va ...
Chapter 6 Outline full
... Solving the equation leads to wave functions, . The wave function gives the shape of the electron’s orbital. • The square of the wave function, , gives the probability of finding the electron. • That is, gives the electron density for the atom. • is called the probability density. Electron ...
... Solving the equation leads to wave functions, . The wave function gives the shape of the electron’s orbital. • The square of the wave function, , gives the probability of finding the electron. • That is, gives the electron density for the atom. • is called the probability density. Electron ...
Nuclear Fission sim
... Why do fission and fusion produce so much energy? • In nuclear reactions, energy comes from converting tiny amounts of mass lost when the bonds between protons and neutrons are broken and made. These bonds are due to the strong force. • The strong force is a thousand times stronger than the electri ...
... Why do fission and fusion produce so much energy? • In nuclear reactions, energy comes from converting tiny amounts of mass lost when the bonds between protons and neutrons are broken and made. These bonds are due to the strong force. • The strong force is a thousand times stronger than the electri ...
Harmonic oscillator - Vibration energy of molecules 1. Definitions
... good approximation of the inter-atomic potential around its minimum, in the vicinity of the equilibrium inter-atomic distance. For real case, it is necessary to consider the 3 dimensions of the space and the coupling between these motions. In this tutorial, we will treat the vibrations of a molecule ...
... good approximation of the inter-atomic potential around its minimum, in the vicinity of the equilibrium inter-atomic distance. For real case, it is necessary to consider the 3 dimensions of the space and the coupling between these motions. In this tutorial, we will treat the vibrations of a molecule ...
Review Unit - hrsbstaff.ednet.ns.ca
... ◘ The modern periodic table arranges the elements in order of increasing atomic number. ◘ Metals are separated from nonmetals by the “staircase line”. metals - shiny, malleable, ductile, conductors of heat and electricity. ◘ The columns are families (groups) of elements having similar chemical prope ...
... ◘ The modern periodic table arranges the elements in order of increasing atomic number. ◘ Metals are separated from nonmetals by the “staircase line”. metals - shiny, malleable, ductile, conductors of heat and electricity. ◘ The columns are families (groups) of elements having similar chemical prope ...
Document
... a) an element which has 5 electrons in each atom b) an element which has 5 electrons in its outer energy level c) an element for which the second energy level is completely filled d) an element which forms ions by gaining only one electron e) how many elements are there in the sixth period? f) the e ...
... a) an element which has 5 electrons in each atom b) an element which has 5 electrons in its outer energy level c) an element for which the second energy level is completely filled d) an element which forms ions by gaining only one electron e) how many elements are there in the sixth period? f) the e ...
Besombes - International Conference on Quantum Dots (QD 2012)
... spin orientation, the recombination of an injected exciton emits a photon with a given energy and polarization. We already demonstrated that a Mn atom embedded in a QD may act as an optically addressable spin based memory [1]. The next steps would be to perform a fast coherent control of an individu ...
... spin orientation, the recombination of an injected exciton emits a photon with a given energy and polarization. We already demonstrated that a Mn atom embedded in a QD may act as an optically addressable spin based memory [1]. The next steps would be to perform a fast coherent control of an individu ...
Name Period Nuclear Study Packet Set 1 1. What subatomic
... 1. What is the half-life of a 100.0 g sample of nitrogen-16 that decays to 12.5 g of nitrogen-16 in 21.6 s? 2. All isotopes of technetium are radioactive, but they have widely varying half-lives. If an 800.0 g sample of technetium-99 decays to 50.0 g of technetium-99 in 639 000 y, what is its ha ...
... 1. What is the half-life of a 100.0 g sample of nitrogen-16 that decays to 12.5 g of nitrogen-16 in 21.6 s? 2. All isotopes of technetium are radioactive, but they have widely varying half-lives. If an 800.0 g sample of technetium-99 decays to 50.0 g of technetium-99 in 639 000 y, what is its ha ...
Let’s talk Chemistry!
... When a chemical reaction and its reverse are occurring at the same time and at the same rate, the reaction has achieved Equilibrium What is the relationship between chemical equilibrium and the rates of forward and the reverse reaction? The forward and reverse reactions must be equal A heterogeneou ...
... When a chemical reaction and its reverse are occurring at the same time and at the same rate, the reaction has achieved Equilibrium What is the relationship between chemical equilibrium and the rates of forward and the reverse reaction? The forward and reverse reactions must be equal A heterogeneou ...
Electrons in Atoms Powerpoint
... As electrons are added to the atom they arrange themselves in orbitals The orbitals are in order of lowest energy (1s) to the highest energy The order of the triangle Fill up in order of energy levels. ...
... As electrons are added to the atom they arrange themselves in orbitals The orbitals are in order of lowest energy (1s) to the highest energy The order of the triangle Fill up in order of energy levels. ...
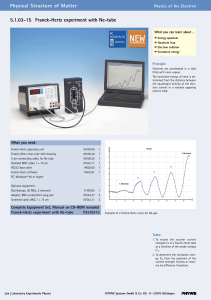
5.1.03-15 Franck-Hertz experiment with Ne
... He also postulated that only those orbits occur for which the angular momentum of the electron is an integral multiple of h/2p, i.e. n*h/2p, where n is an integer and h is Planck’s constant. Bohr’s picture of electrons in discrete states with transitions among those states producing radiation whose ...
... He also postulated that only those orbits occur for which the angular momentum of the electron is an integral multiple of h/2p, i.e. n*h/2p, where n is an integer and h is Planck’s constant. Bohr’s picture of electrons in discrete states with transitions among those states producing radiation whose ...
Rutherford backscattering spectrometry
Rutherford backscattering spectrometry (RBS) is an analytical technique used in materials science. Sometimes referred to as high-energy ion scattering (HEIS) spectrometry, RBS is used to determine the structure and composition of materials by measuring the backscattering of a beam of high energy ions (typically protons or alpha particles) impinging on a sample.