
Fermi-Dirac Statistics
... • E is the energy we are interested in. • T is the temperature at equilibrium. Fermi-Dirac function at Zero Temperature • Plot the function on the board in the limit of T→0. Show the step-wise nature of the transition when E=ΕF. • Note how the step-wise transition at T=0 occurs because E-EF is divid ...
... • E is the energy we are interested in. • T is the temperature at equilibrium. Fermi-Dirac function at Zero Temperature • Plot the function on the board in the limit of T→0. Show the step-wise nature of the transition when E=ΕF. • Note how the step-wise transition at T=0 occurs because E-EF is divid ...
Electronic Structure of Atoms (i.e., Quantum Mechanics)
... the density distribution of the trapped electrons.Their energies and spatial distribution can be quite accurately calculated by solving the classic problem of a quantum mechanical particle in a hard-walled box. Quantum corrals provide us with a unique opportunity to study and visualize the quantum b ...
... the density distribution of the trapped electrons.Their energies and spatial distribution can be quite accurately calculated by solving the classic problem of a quantum mechanical particle in a hard-walled box. Quantum corrals provide us with a unique opportunity to study and visualize the quantum b ...
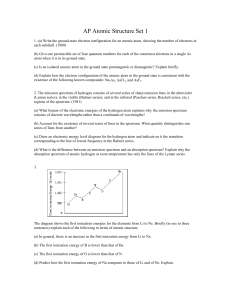
AP Atomic Structure Set 1
... (d) What is the difference between an emission spectrum and an absorption spectrum? Explain why the absorption spectrum of atomic hydrogen at room temperature has only the lines of the Lyman series. ...
... (d) What is the difference between an emission spectrum and an absorption spectrum? Explain why the absorption spectrum of atomic hydrogen at room temperature has only the lines of the Lyman series. ...
Honors Chemistry
... b. How many photons of this light are needed to remove the first electron from each sodium atom that is present in a piece of sodium that has dimensions of 4.36 cm x 36.2 mm x 2.46 in? (DNa = 0.97 g/cm3) ...
... b. How many photons of this light are needed to remove the first electron from each sodium atom that is present in a piece of sodium that has dimensions of 4.36 cm x 36.2 mm x 2.46 in? (DNa = 0.97 g/cm3) ...
Class 39 1
... Because an Li2+ ion has only one orbiting electron, it is like a hydrogen atom with a nuclear charge of +3e. We can use Bohr’s model to find the ground state energy, E1: ...
... Because an Li2+ ion has only one orbiting electron, it is like a hydrogen atom with a nuclear charge of +3e. We can use Bohr’s model to find the ground state energy, E1: ...
Particle on a Sphere
... for a particle →wave functions specified by 2 quantum numbers restricted to values: ...
... for a particle →wave functions specified by 2 quantum numbers restricted to values: ...
Chapter 1: The Mole
... Heart of Chemistry Chemical formulas used. An arrow is used to separate reactants and products. Phase information is sometimes included. Equation carries no implication as to how fast the reaction occurs. ...
... Heart of Chemistry Chemical formulas used. An arrow is used to separate reactants and products. Phase information is sometimes included. Equation carries no implication as to how fast the reaction occurs. ...
Remember Question words
... Atomic structure nucleus (protons, neutrons) shells (electrons) shell = a particular region where electrons can orbit the nucleus of an atom valence electron = an electron in the outermost shell of an atom charges (positive = proton; neutral = neutron; negative = ...
... Atomic structure nucleus (protons, neutrons) shells (electrons) shell = a particular region where electrons can orbit the nucleus of an atom valence electron = an electron in the outermost shell of an atom charges (positive = proton; neutral = neutron; negative = ...
Periodic Trends/Patterns
... nuclear charge. Zeff increases toward nucleus ns > np > nd > nf This explains certain periodic changes observed. ...
... nuclear charge. Zeff increases toward nucleus ns > np > nd > nf This explains certain periodic changes observed. ...
Fourier Transform IR Spectroscopy
... • Outwards from the centreburst the cosine waves cancel and reinforce and the amplitude of the interferogram dies off. ...
... • Outwards from the centreburst the cosine waves cancel and reinforce and the amplitude of the interferogram dies off. ...
unit 7 hw packet File
... Attempt these problems after doing the homework problems in your text. They can serve as a review. Core concepts from unit 2 are mixed in as well. 1.) Ernest Rutherford’s gold foil experiment demonstrated the existence of the (A) alpha particle (B) photon (C) neutron (D) nucleus (E) proton 2.) The r ...
... Attempt these problems after doing the homework problems in your text. They can serve as a review. Core concepts from unit 2 are mixed in as well. 1.) Ernest Rutherford’s gold foil experiment demonstrated the existence of the (A) alpha particle (B) photon (C) neutron (D) nucleus (E) proton 2.) The r ...
Units 1-6
... I can calculate conversions of all types (metric prefix, energy, temperature, density, etc.) including set up, sig figs and units I understand that energy takes different forms, and I can classify energy as potential (chemical, positional, gravitational), kinetic (including temperature) or radiant. ...
... I can calculate conversions of all types (metric prefix, energy, temperature, density, etc.) including set up, sig figs and units I understand that energy takes different forms, and I can classify energy as potential (chemical, positional, gravitational), kinetic (including temperature) or radiant. ...
Helium Atom
... The emission spectra of He consists of a number of series in the visible region of the spectrum as well as in the near & far UV regions. There are twice as many line series as for the alkalis; two principal series in the visible and near UV, as well as two diffuse, two sharp and two fundamental seri ...
... The emission spectra of He consists of a number of series in the visible region of the spectrum as well as in the near & far UV regions. There are twice as many line series as for the alkalis; two principal series in the visible and near UV, as well as two diffuse, two sharp and two fundamental seri ...
Atomic Theory MC 2012
... 10. In the periodic table, as the atomic number increases from 11 to 17, what happens to the atomic radius? (A) It remains constant. (B) It increases only. (C) It increases, then decreases. (D) It decreases only. (E) It decreases, then increases. 11. Which of the following is a correct interpretati ...
... 10. In the periodic table, as the atomic number increases from 11 to 17, what happens to the atomic radius? (A) It remains constant. (B) It increases only. (C) It increases, then decreases. (D) It decreases only. (E) It decreases, then increases. 11. Which of the following is a correct interpretati ...
Name - Red Hook Central Schools
... The Work Function, φ (pronounced “fee”) In actuality, the energy of a photon is never directly proportional to the energy of an ejected electron because the electron must overcome a potential energy barrier (due to a number of quantum and molecular factors). We call this barrier the work function, ...
... The Work Function, φ (pronounced “fee”) In actuality, the energy of a photon is never directly proportional to the energy of an ejected electron because the electron must overcome a potential energy barrier (due to a number of quantum and molecular factors). We call this barrier the work function, ...
Practice MSL Multiple Choice 1. Compared to the charge and mass
... the same charge and a smaller mass the same charge and the same mass an opposite charge and a smaller mass an opposite charge and the same mass ...
... the same charge and a smaller mass the same charge and the same mass an opposite charge and a smaller mass an opposite charge and the same mass ...
Labs - newtunings.com
... 3.1o Stability of an isotope is based on the ratio of neutrons and protons in its nucleus. Although most nuclei are stable, some are unstable and spontaneously decay, emitting radiation. 3.1p Spontaneous decay can involve the release of alpha particles, beta particles, positrons, and/or gamma radiat ...
... 3.1o Stability of an isotope is based on the ratio of neutrons and protons in its nucleus. Although most nuclei are stable, some are unstable and spontaneously decay, emitting radiation. 3.1p Spontaneous decay can involve the release of alpha particles, beta particles, positrons, and/or gamma radiat ...
Chapter 5: The Quantum Mechanical Model of the Atom I. The
... of the atom were _________, and that the amount of energy in the atom was related to the electron s position in the atom. 2. The electrons travel in orbits that are at a fixed distance from the nucleus. ...
... of the atom were _________, and that the amount of energy in the atom was related to the electron s position in the atom. 2. The electrons travel in orbits that are at a fixed distance from the nucleus. ...
Chapter7_1 - Department of Chemistry [FSU]
... The Quantum-Mechanical Model Schrodinger equation: d2% d2% d2% 8&2me [E - V(x,y,z)]%(x,y,z)=0 ...
... The Quantum-Mechanical Model Schrodinger equation: d2% d2% d2% 8&2me [E - V(x,y,z)]%(x,y,z)=0 ...
Rutherford backscattering spectrometry
Rutherford backscattering spectrometry (RBS) is an analytical technique used in materials science. Sometimes referred to as high-energy ion scattering (HEIS) spectrometry, RBS is used to determine the structure and composition of materials by measuring the backscattering of a beam of high energy ions (typically protons or alpha particles) impinging on a sample.



















![Chapter7_1 - Department of Chemistry [FSU]](http://s1.studyres.com/store/data/016128835_1-aea3c1aec04363d6cbf538e8faf80e45-300x300.png)