Atomic Radii Answers File
... charge has not changed. However, now the nucleus is attracting one less electron so the remaining ones are pulled in closer. When an atom gains an electron to form a negative ion, the nuclear charge has not changed. However, now the nucleus is attracting one more electron so they are not pulled as s ...
... charge has not changed. However, now the nucleus is attracting one less electron so the remaining ones are pulled in closer. When an atom gains an electron to form a negative ion, the nuclear charge has not changed. However, now the nucleus is attracting one more electron so they are not pulled as s ...
Introduction_to_Geochemistry_Pre-Lecture_Quiz
... detach the loosest electron from atoms of that element. (e) All alkali metals have similar chemical properties. (f) Alkali earths have one electron in the outer shell. (g) Electronegativity is the amount of negative charge on an atom. (h) Ca has a valency of 2. (i) True ionic bonds are unknown and a ...
... detach the loosest electron from atoms of that element. (e) All alkali metals have similar chemical properties. (f) Alkali earths have one electron in the outer shell. (g) Electronegativity is the amount of negative charge on an atom. (h) Ca has a valency of 2. (i) True ionic bonds are unknown and a ...
Test 1 Guide
... 1) Neutrons have almost no mass (in amu) and no charge. 2) The fact that 2 different elements may have atoms with the same atomic mass violates Dalton’s atomic theory. 3) Deuterium differs from hydrogen in the number of protons it has. 4) In the quantum mechanical model of the atom, electrons are co ...
... 1) Neutrons have almost no mass (in amu) and no charge. 2) The fact that 2 different elements may have atoms with the same atomic mass violates Dalton’s atomic theory. 3) Deuterium differs from hydrogen in the number of protons it has. 4) In the quantum mechanical model of the atom, electrons are co ...
Chemical Building Blocks
... How many neutrons are there in the 90Sr nucleus? A rubidium isotope has 50 neutrons. What is its mass no.? How many neutrons does 90Mo have? How many neutrons are in bromine-81? Which of the following isotopes are of the same element? Name the isotopes. ...
... How many neutrons are there in the 90Sr nucleus? A rubidium isotope has 50 neutrons. What is its mass no.? How many neutrons does 90Mo have? How many neutrons are in bromine-81? Which of the following isotopes are of the same element? Name the isotopes. ...
Total view of the AFM
... • There are different types – Ion Microprobe, TOF-SIMS, and Quadrupole SIMS. The first two are more important: the first is also called dynamic SIMS where a complete depth profile can be done and uses q/m ratio to separate ions, and the 2nd used for static SIMS as only a few monolayers are removed, ...
... • There are different types – Ion Microprobe, TOF-SIMS, and Quadrupole SIMS. The first two are more important: the first is also called dynamic SIMS where a complete depth profile can be done and uses q/m ratio to separate ions, and the 2nd used for static SIMS as only a few monolayers are removed, ...
Unit 1 Inorganic Flashcards
... surrounding molecules or ions by dative covalent bonds (also known as coordinate bonds). ...
... surrounding molecules or ions by dative covalent bonds (also known as coordinate bonds). ...
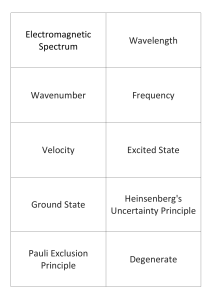
Electromagnetic Spectrum Wavelength Wavenumber Frequency
... surrounding molecules or ions by dative covalent bonds (also known as coordinate bonds). ...
... surrounding molecules or ions by dative covalent bonds (also known as coordinate bonds). ...
No Slide Title
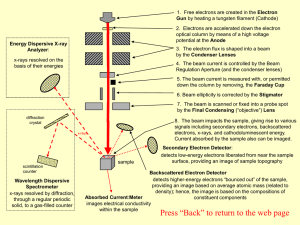
... down the column by removing, the Faraday Cup 6. Beam ellipticity is corrected by the Stigmator 7. The beam is scanned or fixed into a probe spot by the Final Condensing (“objective”) Lens ...
... down the column by removing, the Faraday Cup 6. Beam ellipticity is corrected by the Stigmator 7. The beam is scanned or fixed into a probe spot by the Final Condensing (“objective”) Lens ...
Combining and Choosing Analytical Techniques
... volumetric analysis, UV-visible spectroscopy, HPLC, infrared spectroscopy and NMR spectroscopy. Each technique will give different information. We can even combine some of the techniques. ...
... volumetric analysis, UV-visible spectroscopy, HPLC, infrared spectroscopy and NMR spectroscopy. Each technique will give different information. We can even combine some of the techniques. ...
Review - ND
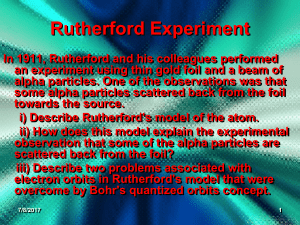
... In 1911, Rutherford and his colleagues performed an experiment using thin gold foil and a beam of alpha particles. One of the observations was that some alpha particles scattered back from the foil towards the source. i) Describe Rutherford's model of the atom. ii) How does this model explain the ex ...
... In 1911, Rutherford and his colleagues performed an experiment using thin gold foil and a beam of alpha particles. One of the observations was that some alpha particles scattered back from the foil towards the source. i) Describe Rutherford's model of the atom. ii) How does this model explain the ex ...
Rutherford backscattering spectrometry
Rutherford backscattering spectrometry (RBS) is an analytical technique used in materials science. Sometimes referred to as high-energy ion scattering (HEIS) spectrometry, RBS is used to determine the structure and composition of materials by measuring the backscattering of a beam of high energy ions (typically protons or alpha particles) impinging on a sample.