* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Combining and Choosing Analytical Techniques
Electron paramagnetic resonance wikipedia , lookup
Astronomical spectroscopy wikipedia , lookup
Electrochemistry wikipedia , lookup
Scanning tunneling spectroscopy wikipedia , lookup
Membrane potential wikipedia , lookup
Electron scattering wikipedia , lookup
Ultrafast laser spectroscopy wikipedia , lookup
Chemical bond wikipedia , lookup
Physical organic chemistry wikipedia , lookup
Gamma spectroscopy wikipedia , lookup
Electron configuration wikipedia , lookup
Particle-size distribution wikipedia , lookup
Reflection high-energy electron diffraction wikipedia , lookup
Stability constants of complexes wikipedia , lookup
Vibrational analysis with scanning probe microscopy wikipedia , lookup
Auger electron spectroscopy wikipedia , lookup
Nanofluidic circuitry wikipedia , lookup
Isotopic labeling wikipedia , lookup
Atomic absorption spectroscopy wikipedia , lookup
Ionic compound wikipedia , lookup
Metastable inner-shell molecular state wikipedia , lookup
X-ray photoelectron spectroscopy wikipedia , lookup
Mössbauer spectroscopy wikipedia , lookup
Two-dimensional nuclear magnetic resonance spectroscopy wikipedia , lookup
Atomic theory wikipedia , lookup
Chemical imaging wikipedia , lookup
Ultraviolet–visible spectroscopy wikipedia , lookup
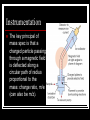
Combining and Choosing Analytical Techniques Chapter 8 Mass Spectrometry One of the most common and useful analytical tools used in combination with other techniques is mass spectrometry. Mass Spec can be used for: Quantitative analysis – a sophisticated and very sensitive detector that can measure how much of a substance is present Qualitative – to provide a unique fingerprint of a substance, this can be used to identify the substance from an on-line database or to give information about the structure of a new or unknown compound. Instrumentation The key principal of mass spec is that a charged particle passing through a magnetic field is deflected along a circular path of radius proportional to the mass: charge ratio, m/e (can also be m/z). Instrumentation The sample as a gas enters the evacuated tube. Positive ions are formed in the ionisation chamber when an electron beam dislodges electrons from the sample atoms The positive ions are accelerated by an electric field The ions enter a magnetic field perpendicular to their path. This causes the ions to move in a curved path with a radius that depends upon the m/z ratio of the ions. Only ions moving in a curved path of a particular radius corresponding to a fixed m/z ratio will reach the collector The collector measures the current due to the ions reaching the detector and the data is recorded as a mass spectrum. How the spectrum is formed A molecular substance can give a range of peaks in the spectrum. Two factors cause the many peaks in the spectrum: The fragmentation of the molecules into a large number of different positive ions The occurrence of different isotopes of the atoms that make up the molecules Fragmentation The high energy electron beam can knock just one electron from the molecule to form a positive ion: M + e- → M+ + 2eM+ is called the parent molecular ion. The parent molecular ion is a radical, with one unpaired electron. It is chemically unstable and so will often break into smaller fragments Fragmentation Look at page 112. What happens to ethanoic acid. When an ion fragments into two smaller parts, one ion will retain the electron to become the uncharged free radical and the other is positively charged. Only the positive ions reach the detector Isotope effects In the same spectrum additional peaks can be formed due to the occurrence of different isotopes of an element. Chlorine for example has two isotopes. 35Cl (76% abundance) and 37Cl (24% abundance) Interpreting Mass Spectra The height of the peak gives the relative concentration of the ions present. The highest peak is assigned an intensity of 100% and all other peaks are measured relative to the base peak. The relative intensities of the ions depend on: The energy of the bombarding electrons The stability of the ion fragments formed The ease with which ions can lose atoms Your Turn Page 114 Questions 1-5 Combined Techniques A chemist presented with a sample will usually have more than one technique to call upon. For example acetylsalicylic acid, the active ingredient in aspirin, can be analysed by volumetric analysis, UV-visible spectroscopy, HPLC, infrared spectroscopy and NMR spectroscopy. Each technique will give different information. We can even combine some of the techniques. Combined Techniques Many instruments combine two techniques to provide more detailed and rapid information about a sample. The most commonly used combined techniques are gas chromatography-mass spectrometry (GC-MS) and HPLC-mass spectrometry (HPLC-MS) Chromatography and Mass Spectrometry The advantage of combining these two techniques is that chromatography can separate a complex sample into any number of components and each one can be positively identified through Mass spec. These are used all the times in forensic analysis. As individual components of the sample elute leave the chromatography column they enter the ionisation chamber of the mass spec. Things to consider when choosing a technique Chemical Considerations Analyte Is the sample a metal or a non-metal? Is it coloured or transparent? What functional groups are present? Is it volatile? Is it soluble in water or other solvents Chemical Considerations Sample How much is available? The concentration of the analyte in the sample. The physical state of the sample What else is present in the sample can it interfere Economic Considerations Time Cost of Equipment Expertise Quality of Data Your Turn Page 122 Question 12 and 13 Worksheet